A baby is expected to have his eyesight saved after a new screening tool picked up a rare eye cancer which may have otherwise gone undiagnosed.
Just four weeks after he was born at Sheffield Teaching Hospital in April, Freddie was diagnosed with…

A baby is expected to have his eyesight saved after a new screening tool picked up a rare eye cancer which may have otherwise gone undiagnosed.
Just four weeks after he was born at Sheffield Teaching Hospital in April, Freddie was diagnosed with…

Posted: 17 October 2025
Chimeric Therapeutics (ASX:CHM) has signed a Letter of Intent (LOI) with Viral Vector Manufacturing Facility Pty Ltd (VVMF) to establish a strategic partnership focused on the development and Good Manufacturing Practice (GMP) production of Lentiviral vectors in Australia.
Under the agreement, VVMF will support process development, technology transfer and GMP-grade manufacturing of Lentiviral vectors for Chimeric’s clinical-stage chimeric antigen receptor T-cell (CAR-T) therapy programme. Viral vectors are essential in producing CAR-T therapies, which are revolutionising cancer treatment worldwide.
“We’re pleased to partner with VVMF as we continue to advance our CAR-T cell therapy programmes,” said Chimeric Therapeutics CEO Dr Rebecca McQualter. “Having access to local, GMP-grade viral vector manufacturing not only strengthens our supply chain but also supports the broader goal of building world-class advanced therapy capabilities here in Australia.”
VVMF CEO Stephen Thompson said the collaboration would strengthen sovereign manufacturing and create high-value jobs in Western Sydney. “This collaboration allows us to demonstrate our capability to develop and manufacture GMP-grade viral vectors for the global cell and gene therapy marketplace,” he said.
The agreement was described as a milestone for Australia’s growing advanced manufacturing sector and the development of Advanced Therapy Medicinal Products (ATMPs) — innovative medicines derived from genes, cells, or engineered tissues. These therapies are opening new possibilities for treating cancer, neurodegenerative, and cardiovascular diseases.
Supported by strong R&D incentives, a pragmatic regulatory environment and a mature clinical trial ecosystem, Australia is well positioned to become a global hub for advanced therapy development and manufacturing.
Find out more here.

Joanne WrittleWest Midlands health correspondent
 BBC
BBCA family has described their heartbreak and anger after a mother was twice sent home…
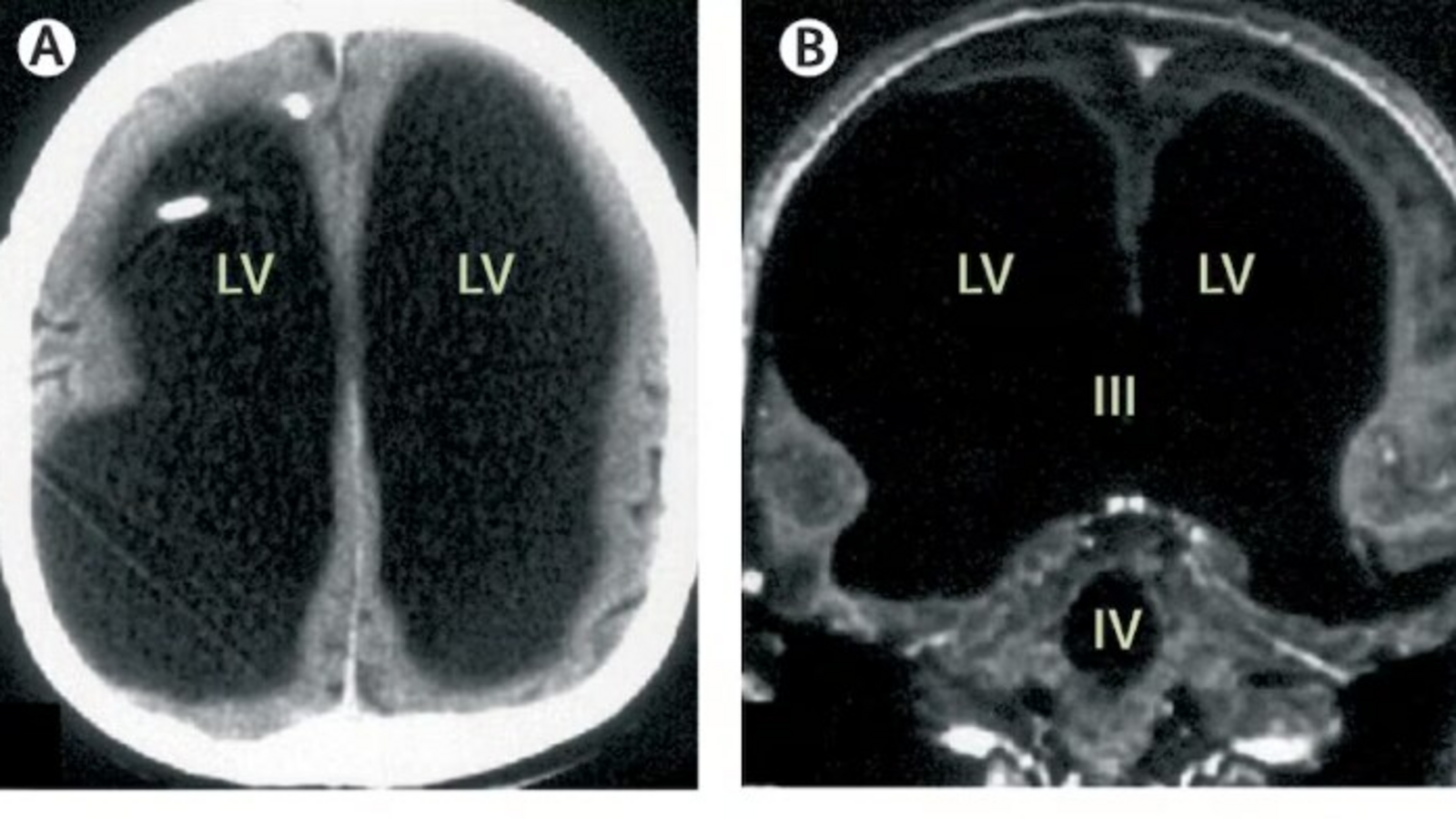
A rare medical case, first described in The Lancet in 2007, highlights the brain’s remarkable adaptability and neuroplasticity. Despite losing 90 percent of his brain, the then 44-year-old French man was reportedly able to lead a relatively…

Underweight children cost the NHS as much as those with obesity, a new study has found.
The University of Oxford findings concluded the health service spends about £340m in additional costs annually due to weight-related problems in children.
Continue Reading

Chrissie Reidy and
Craig BuchanSouth East
 Chrissie Reidy/BBC
Chrissie Reidy/BBCRelatives of victims in a fatal Air India plane crash say the Foreign Office has…

By Dr Mariangela Pellegrini, Lora Ruth Wogu and Marie-Claire Kofi, Co-Chairs of the Sickle Cell Transitions Policy Lab
In recent years, legislation and policies have transformed the rare disease landscape in the EU, improving patient access to…

On the International Day for the Eradication of Poverty, the United Nations Development Programme (UNDP) renews its resolve to eradicate poverty and advance shared prosperity. This year’s theme, “Ending social and institutional…

Joe CampbellSouth of England and
Rachel Russell
 Joe Campbell/BBC
Joe Campbell/BBCParents are being encouraged to get their toddlers involved in a new clinical trial aimed at helping lower the age…

K-pop is a genre of music that – brace yourself – originated in South Korea and blends pop, hip-hop, R&B and electronica, all filtered through an east Asian prism. The music tends to be…