Given that polygenic risk doesn’t change and only needs to be measured once, researchers advocate greater use.
Combining a polygenic risk score (PRS) with traditional biomarkers can help clinicians better estimate a patient’s risk of…
Combining a polygenic risk score (PRS) with traditional biomarkers can help clinicians better estimate a patient’s risk of…

Glenn Close has joined the cast of the Spanish drama The Black Ball (La Bola Negra), the new film from the writing-directing team of Javier Calvo and Javier Ambrossi (Veneno, The Messiah). Produced by Movistar Plus+ and Suma Content Films,…

Avoiding artificial light at nighttime could lower the risk of cardiovascular disease, research suggests.
The study,…
The fight against malaria has stalled after two decades of progress, with climate change and population growth among factors threatening a resurgence of the potentially fatal disease, campaigners said Tuesday.
Insufficient funding for…
This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!

GSK plc (LSE/NYSE: GSK) today announced the US Food and Drug Administration (FDA) has approved Blenrep (belantamab mafodotin-blmf) in combination with bortezomib and dexamethasone (BVd) for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least two prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory (IMID) agent.
The Blenrep approval is supported by data from the pivotal DREAMM-7 phase III trial. In patients who had two or more prior lines of therapy (3L+), including a PI and an IMID, Blenrep in combination demonstrated a clinically meaningful 51% reduction in the risk of death [HR 0.49, 95% confidence interval (CI): 0.32-0.76] and a tripled median progression-free survival (PFS) of 31.3 months [95% CI: 23.5-NR)] versus 10.4 months [95% CI: 7.0-13.4] for a daratumumab-based triplet (DVd) [HR 0.31, 95% CI: 0.21-0.47]. The safety and tolerability profiles of the Blenrep combination were broadly consistent with the known profiles of the individual agents.2
Tony Wood, Chief Scientific Officer, GSK, said: “Today’s FDA approval of Blenrep is another significant milestone, providing potential for superior efficacy, including overall survival, to US patients. There is an urgent need for new and novel therapies, as nearly all patients with multiple myeloma experience relapse and re-treating with the same mechanism of action often leads to suboptimal outcomes. As the only anti-BCMA agent that can be administered across healthcare settings, including in community centres where 70% of patients receive care, Blenrep fulfils a major patient need. We believe Blenrep can redefine treatment for patients with multiple myeloma in all parts of the world, and we are accelerating its development in earlier lines of therapy to support its use across all stages of this difficult-to-treat cancer.”
Working closely with the FDA, Blenrep is available through a new, streamlined Risk Evaluation and Mitigation Strategy (REMS). The new REMS supports appropriate use and patient safety while reducing administrative burden through simplified patient forms, removal of duplicative checklists and efficient communication between HCPs and either optometrists or ophthalmologists monitoring eye care. GSK will also offer Together with GSK, an optional patient support programme available to all US patients prescribed Blenrep.
Data from the DREAMM (DRiving Excellence in Approaches to Multiple Myeloma) clinical trial programme will be submitted to the National Comprehensive Cancer Network (NCCN) guidelines this year. Recent results from the DREAMM studies, alongside emerging real-world evidence, provide a growing body of data for Blenrep.5,6
Sagar Lonial, MD, Chief Medical Officer, Winship Cancer Institute of Emory University in Atlanta, Georgia, Chair of Emory Department of Hematology and Medical Oncology, said: “With the approval of Blenrep, we now have a community-accessible BCMA-targeting agent with the potential to improve outcomes for patients following two or more prior lines of treatment, where options are limited. This approval marks an important advance in the US relapsed/refractory treatment landscape.”
Michael Andreini, President and Chief Executive Officer of the Multiple Myeloma Research Foundation and the Multiple Myeloma Research Consortium, said: “The reality for most patients with multiple myeloma is a relentless cycle of remission and relapse, as their disease becomes refractory to treatments. Patients urgently need more effective treatment options that can offer more quality time with their loved ones. We see the potential for Blenrep in combination to help patients achieve this.”
GSK is advancing the DREAMM clinical programme to demonstrate Blenrep’s potential benefit in earlier lines of treatment. Follow-up continues for overall survival (OS) in both DREAMM-7 and DREAMM-8 with data expected in early 2028, including in patients who have received only one prior line of therapy. DREAMM-10, a phase III trial in newly diagnosed transplant-ineligible patients, which represent over 70% of patients starting therapy, was initiated in Q4-2024.4 Interim efficacy and safety data for Blenrep as a first line treatment are expected in early 2028 with enrolment expanded to US sites to increase US patient representation in the study population. GSK continues to work with the FDA for US patients.
Blenrep combinations are approved in 2L+ relapsed or refractory multiple myeloma in the European Union7, UK8, Japan9, Canada, Switzerland and Brazil. Applications are currently under review in other markets globally, including China10 where the application is based on the results of DREAMM-7 and has been granted Breakthrough Therapy Designation and Priority Review.
Multiple myeloma is the third most common blood cancer globally and is generally considered treatable but not curable.11,12 There are approximately 180,000 new cases of multiple myeloma diagnosed globally each year. Research into new therapies is needed as multiple myeloma commonly becomes refractory to available treatments.13 Many patients with multiple myeloma, including approximately 70% in the US, are treated in a community cancer setting, leaving an urgent need for new, effective therapies with manageable side effects that can be administered outside of an academic centre.3,15,16
Blenrep is a monoclonal ADC (antibody-drug conjugate) comprising a humanised BCMA (B-cell maturation antigen) conjugated to the cytotoxic agent auristatin F via a non-cleavable linker. The drug linker technology is licensed from Seagen Inc.; the monoclonal antibody is produced using POTELLIGENT Technology licensed from BioWa Inc., a member of the Kyowa Kirin Group.
In the US, Blenrep is indicated in combination with bortezomib and dexamethasone (BVd) for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least two prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent.
Please see accompanying US Prescribing Information which will soon be available here17.
DREAMM-7 is a multicentre, open-label, randomised phase III clinical trial evaluating the efficacy and safety of belantamab mafodotin combined with bortezomib plus dexamethasone (BVd) compared to daratumumab combined with bortezomib plus dexamethasone (DVd) in patients with relapsed or refractory multiple myeloma who previously were treated with at least one prior line of multiple myeloma therapy, with documented disease progression during or after their most recent therapy. The trial enrolled 494 participants who were randomised 1:1 to receive either BVd or DVd. Belantamab mafodotin was administered at a dose of 2.5mg/kg intravenously every three weeks in combination for the first eight cycles and then continued as a single agent. The primary endpoint was PFS as per an independent review committee, with secondary endpoints including OS, duration of response (DOR), and minimal residual disease (MRD) negativity rate as assessed by next-generation sequencing. Other secondary endpoints include overall response rate (ORR), safety, and patient reported and quality of life outcomes.
PFS results17 were presented at the American Society of Clinical Oncology (ASCO) Plenary Series in February 2024 and published in the New England Journal of Medicine. OS results18 were presented at the American Society of Hematology (ASH) Annual Meeting in December 2024.
DREAMM-10 is a multicentre, open-label, randomised phase III clinical trial in newly diagnosed transplant ineligible patients with multiple myeloma, evaluating belantamab mafodotin plus lenalidomide and dexamethasone (BRd) versus daratumumab plus lenalidomide and dexamethasone (DRd).4
Our ambition in oncology is to help increase overall quality of life, maximise survival and change the course of disease, expanding from our current focus on blood and women’s cancers into lung and gastrointestinal cancers, as well as other solid tumours. This includes accelerating priority programmes such as antibody-drug conjugates targeting B7-H3 and B7-H4, and IDRX-42, a highly selective KIT tyrosine kinase inhibitor.
GSK is a global biopharma company with a purpose to unite science, technology, and talent to get ahead of disease together. Find out more at gsk.com.
GSK cautions investors that any forward-looking statements or projections made by GSK, including those made in this announcement, are subject to risks and uncertainties that may cause actual results to differ materially from those projected. Such factors include, but are not limited to, those described in the “Risk Factors” section in GSK’s Annual Report on Form 20-F for 2024, and GSK’s Q2 Results for 2025.

Aston Villa boss Unai Emery said his side have to become more clinical from the penalty spot after a shock Europa League defeat to Dutch minnows Go Ahead Eagles.
Emiliano Buendía missed a late spot-kick as Villa…

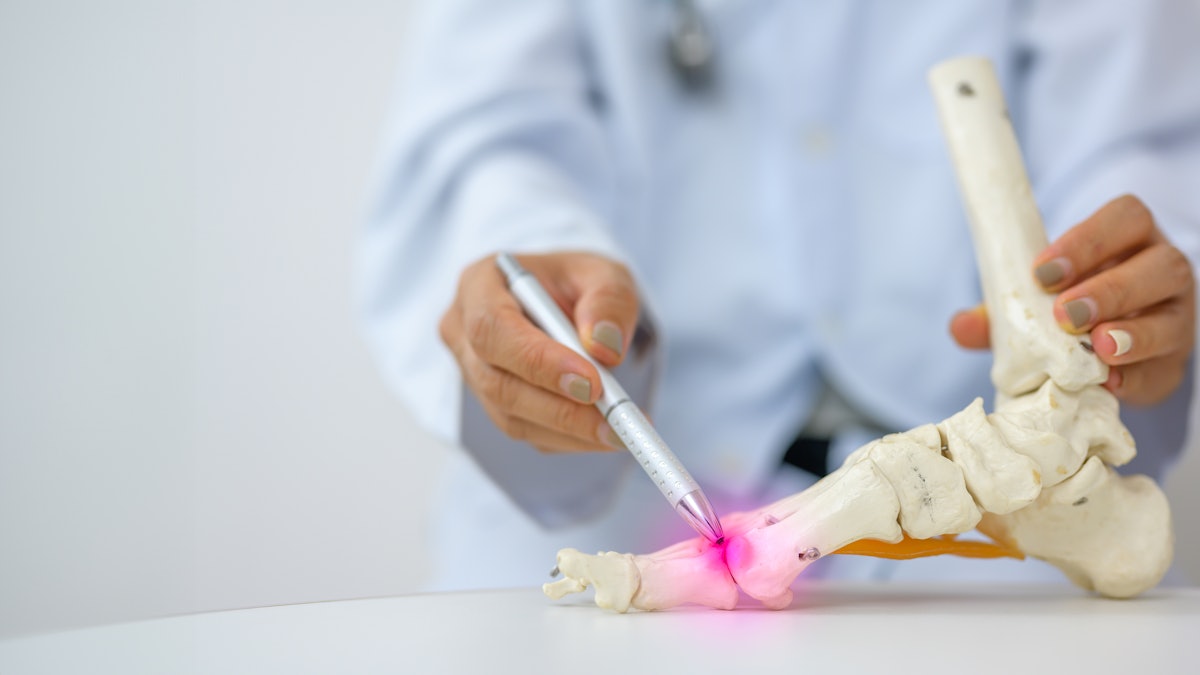
Interventional radiologists in Turkey have reported success with an emerging minimally invasive procedure for treating chronic pain in toe joints due to inflammatory arthritis, gout, or osteoarthritis.
The procedure is called transarterial…

The US Department of Agriculture’s (USDA’s) Animal and Plant Health Inspection Service (APHIS) announced another flurry of highly pathogenic avian flu detections in waterfowl and other wild birds from across the country.
APHIS also reported that…