AOL is part of the Yahoo family of brands.
-
…
AOL is part of the Yahoo family of brands.

First Period
It was the visitors who came out of the traps quickest and made Jackson Whistle work. Vlastimil Dostalek rattled the crossbar before Whistle made a good blocker save from Jaka Sturm. Momentum would swing back towards the…

Delete all these texts.
getty
Text attacks on Android and iPhone owners in America, Europe and elsewhere are totally out of control. This billion-dollar industry now defrauds millions of smartphone owners. The malicious technologies that execute…

KEYNOTE-905/EV-303 (NCT03924895), a phase 3, open-label, randomized trial, evaluated perioperative enfortumab vedotin (EV) plus pembrolizumab (pembro) compared with surgery alone in patients with muscle-invasive bladder cancer (MIBC) who were ineligible for or declined cisplatin-based chemotherapy.
Presented by Prof. Christof Vulsteke at the ESMO Congress 2025, the study demonstrated that adding EV + pembro to radical cystectomy with pelvic lymph node dissection resulted in significant improvements in event-free survival (EFS), overall survival (OS), and pathologic complete response (pCR). These findings mark a major advance for cisplatin-ineligible MIBC, establishing a potential new perioperative standard of care.
Radical cystectomy with pelvic lymph node dissection (RC + PLND) remains the standard of care for patients with muscle-invasive bladder cancer. However, nearly half of these patients are ineligible for cisplatin-based chemotherapy due to renal dysfunction, frailty, or comorbidities, leaving a substantial population without effective perioperative options.
Previous studies have shown limited benefit from surgery alone in this setting, underscoring the unmet need for alternative strategies. Enfortumab vedotin, an antibody–drug conjugate targeting Nectin-4, combined with pembrolizumab, has demonstrated potent antitumor activity and a favorable safety profile in metastatic urothelial cancer, providing a strong rationale for evaluation in the perioperative context.
KEYNOTE-905/EV-303 is a randomized, open-label, phase 3 trial that enrolled adults with MIBC (T2–T4aN0M0 or T1–T4aN1M0) who were cisplatin-ineligible per Galsky criteria or declined cisplatin.
Participants were randomized 1:1 to receive:
The primary endpoint was event-free survival (EFS) by blinded independent central review (BICR).Key secondary endpoints included overall survival (OS), pathologic complete response (pCR), and safety.
A total of 344 participants were randomized between December 2020 and June 2024 (170 to EV + pembro; 174 to control). Over 80% were cisplatin-ineligible, and the median follow-up was 25.6 months (range 11.8–53.7).The combination regimen produced statistically significant and clinically meaningful improvements across all efficacy endpoints:
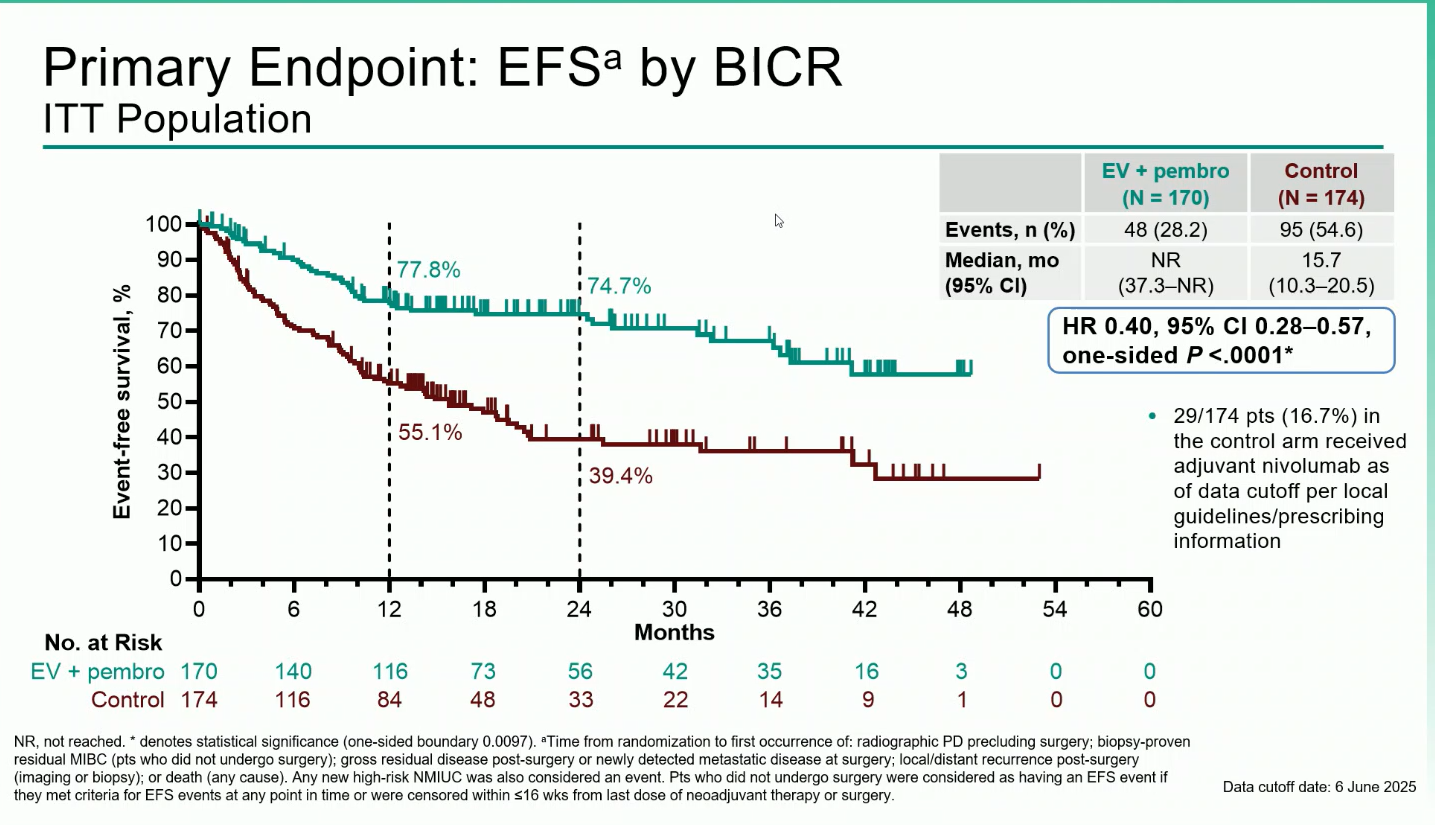
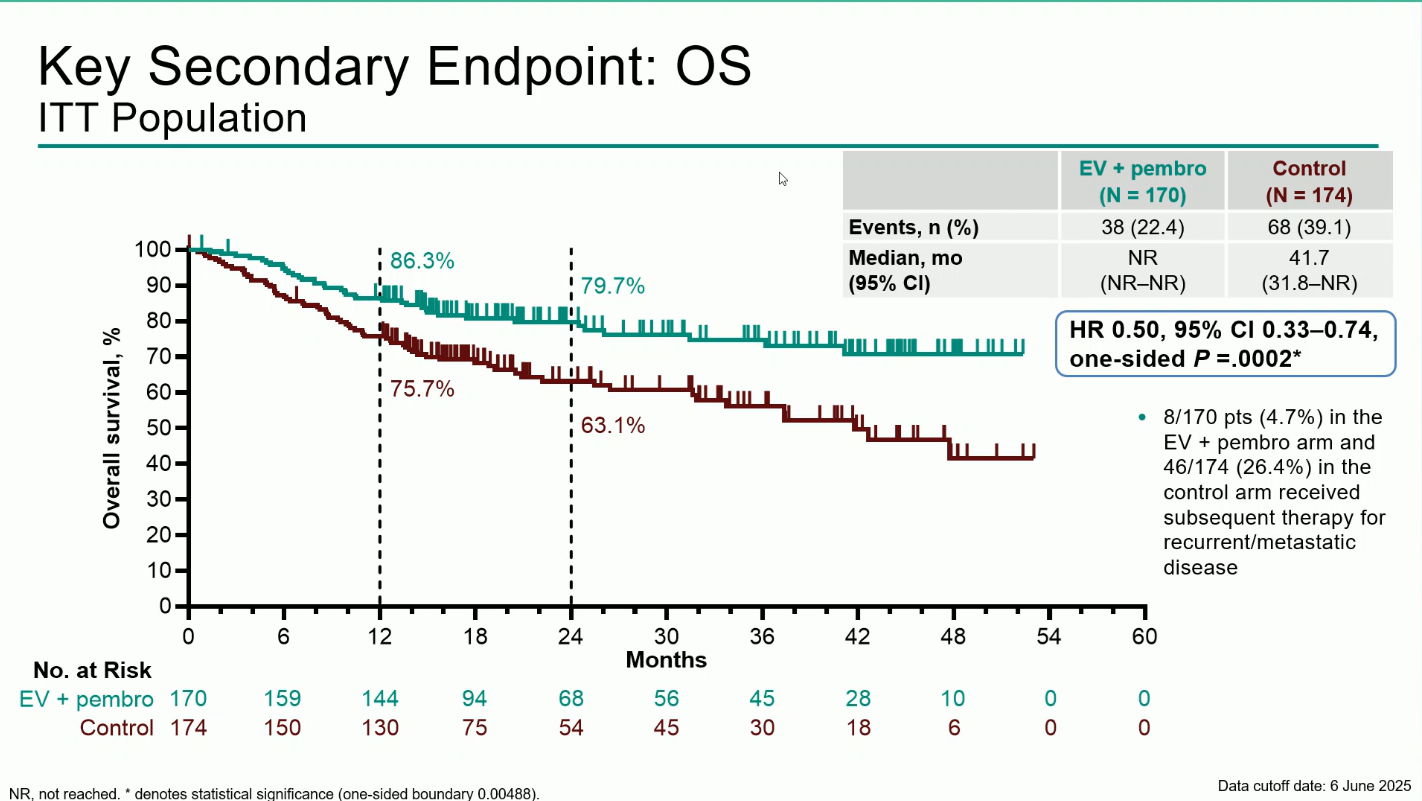
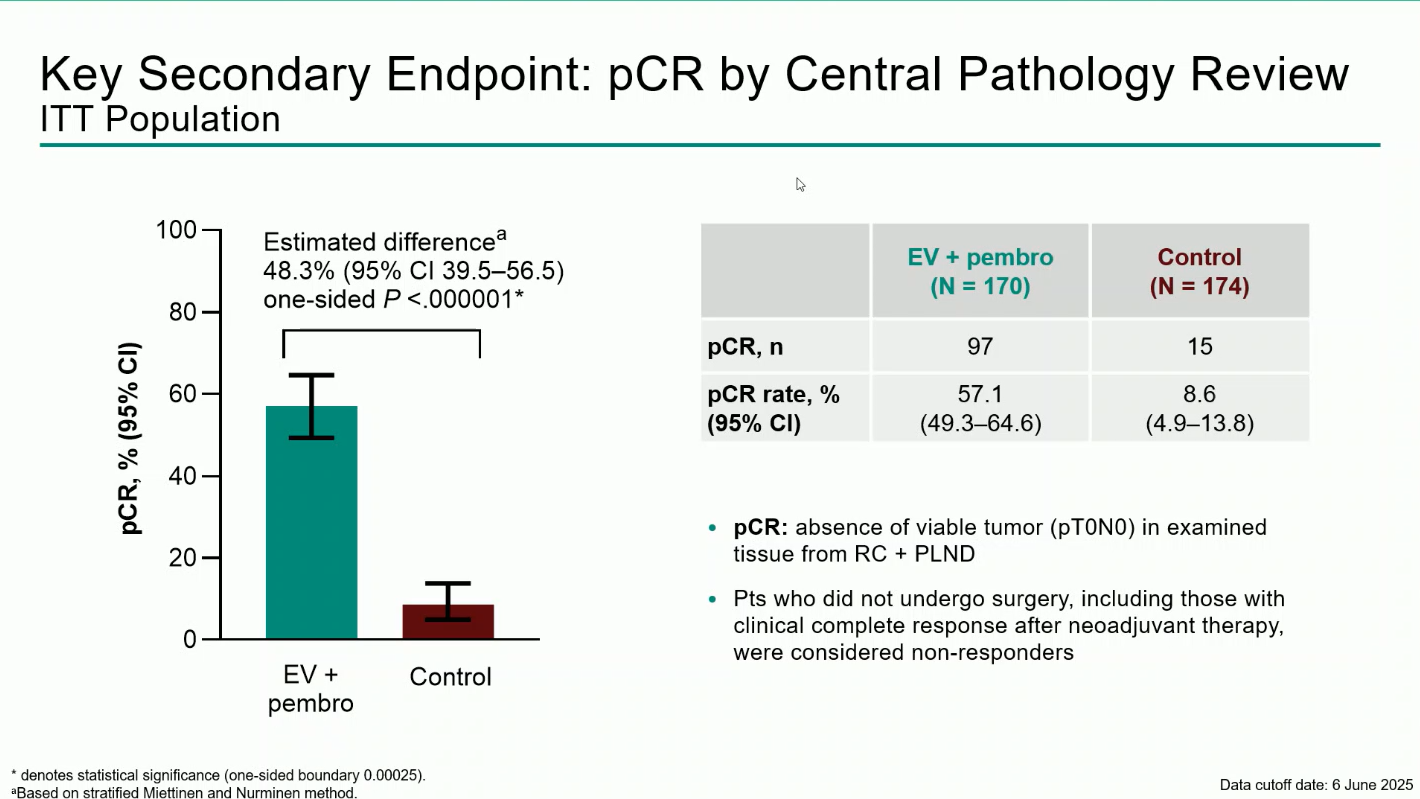
Treatment-emergent adverse events (TEAEs) occurred in nearly all patients (100% in EV + pembro vs 64.8% in control). Grade ≥3 events were reported in 71.3% and 45.9%, respectively. The most frequent grade ≥3 adverse events of special interest were severe skin reactions (11.4%) related to pembrolizumab and cutaneous toxicity (10.8%) associated with enfortumab vedotin.
Despite the high incidence of AEs, the safety profile remained manageable and consistent with prior studies, and no new safety signals were identified.
The KEYNOTE-905/EV-303 trial demonstrated that adding perioperative enfortumab vedotin plus pembrolizumab to standard surgery significantly improved event-free survival, overall survival, and pathologic complete response rates in patients with cisplatin-ineligible MIBC.
These findings establish EV + pembrolizumab as the first perioperative regimen to improve outcomes versus RC + PLND alone in this population, offering a potential new standard of care.
You can read the full abstract here.
For decades, scientists have known that lead is bad news for brains. It messes with brain development, lowers intelligence, and causes emotional and behavioral problems.
But no one expected to find proof of lead exposure in ancient hominids –…

Red Cat Holdings, Inc. (NASDAQ:RCAT) is one of the stocks Jim Cramer was focused on recently. When a caller asked about the stock during the lightning round, Cramer said:
“Okay, so this is a Ben Stoto favorite, not really. It’s a drone company. We are on the fence about buying drone companies that aren’t making money.”
Pixabay/Public Domain
Red Cat Holdings, Inc. (NASDAQ:RCAT) develops drone systems and control technologies for military, government, and commercial use. During the February 18 episode, Cramer mentioned the stock and remarked:
“This one just happens to be a personal favorite of our chief scientist, Ben Stoto. We talk about Red Cat a lot. It’s a data analytics company, and you know what? I’m going to tell you, you can buy it. You really can. Because if it doubled, you’d feel like an idiot for not buying Red Cat.”
It is worth noting that since the above comment was made, the company’s stock gained around 65%.
While we acknowledge the potential of RCAT as an investment, we believe certain AI stocks offer greater upside potential and carry less downside risk. If you’re looking for an extremely undervalued AI stock that also stands to benefit significantly from Trump-era tariffs and the onshoring trend, see our free report on the best short-term AI stock.
READ NEXT: 30 Stocks That Should Double in 3 Years and 11 Hidden AI Stocks to Buy Right Now.
Disclosure: None. This article is originally published at Insider Monkey.