On the gut health side of TikTok there’s a genre of videos where people jokingly celebrate having a…

Phase three of the Midnight Alpha is here and with it, players in the test can continue to level through levels 83 to 88, explore the Harandar and Eversong Woods Zones, take on two new Delves and one new dungeon, and more.
Midnight Alpha…
RARITAN, NEW JERSEY, October 15, 2025 – Johnson & Johnson today announced plans to showcase 16 presentations at the
European Society for Medical Oncology (ESMO) 2025 Congress, spotlighting advances across its industry-leading solid tumor pipeline—including head and neck, lung, bladder, and prostate cancers. These data showcase accelerating momentum and the Company’s commitment to transforming care in some of the most challenging and underserved cancers.
“We’ve entered a new era for Johnson & Johnson in oncology, delivering the latest bold advances in solid tumors,” said Yusri Elsayed, M.D., M.H.Sc., Ph.D., Global Therapeutic Area Head, Oncology, Johnson & Johnson Innovative Medicine. “We’re advancing the next generation of treatments and resetting expectations across bladder, prostate, colorectal, head and neck and EGFR-mutated lung cancer.”
“These results highlight the promise of our innovation, from novel mechanisms that harness the immune system to organ-preserving approaches that could change standards of care,” said Henar Hevia, Ph.D., Senior Director, EMEA Therapy Area Head, Oncology, Johnson & Johnson Innovative Medicine. “Our ambition is to translate our deep expertise in hematology into tangible progress in solid tumors, delivering therapies that not only extend survival but also preserve quality of life for patients and families.”
Highlights include studies across four different solid tumor types:
All Johnson & Johnson company-sponsored abstracts to be presented are listed below:
| Head & Neck Cancer | |
| Subcutaneous Amivantamab | |
| Oral Presentation | |
|
October 19, 2025 4:30 – 6:00 PM CEST Abstract #1327MO |
Amivantamab in Recurrent/Metastatic Head & Neck Squamous Cell Cancer (HNSCC) After Disease Progression on Checkpoint Inhibition and Chemotherapy: Results From the Phase 1b/2 OrigAMI-4 Study |
| Poster Presentation | |
|
October 20, 2025 12 – 12:45 PM CEST Abstract #1352P |
Amivantamab Plus Paclitaxel in Recurrent/Metastatic (R/M) Head & Neck Squamous Cell Cancer (HNSCC) After Disease Progression on Checkpoint Inhibition: Identification of the Recommended Combination Dose From the Phase 1b/2 OrigAMI-4 Study |
| Lung Cancer | |
| Subcutaneous Amivantamab | |
| Poster/eTiP Presentations | |
|
October 18, 2025 12 – 12:45 PM CEST Abstract #1960P |
Subcutaneous (SC) Amivantamab (Ami) Plus Chemotherapy (Chemo) in EGFR-Mutant (EGFRm) Advanced Non-Small Cell Lung Cancer (NSCLC) After Disease Progression on Osimertinib (Osi) |
| Abstract #2082eTIP | COPERNICUS: A Multinational Pragmatic Phase 2 Trial of Subcutaneous (SC) Amivantamab (Ami) in Common EGFR-Mutated (cEGFRm) NSCLC |
| Poster Presentation | |
|
October 18, 2025 12 – 12:45 PM CEST Abstract #1826P |
Real-World Utilization and Outcomes With PACIFIC Regimen in Stage III Unresectable Non-Small Cell Lung Cancer Patients: Results From a Multicenter US Database |
| Bladder Cancer | |
| Gemcitabine Intravesical System | |
| Oral Presentation | |
|
October 17, 2025 4 – 5:30 PM CEST Abstract #LBA112 |
Neoadjuvant Gemcitabine Intravesical System + Cetrelimab (CET) or CET Alone in Patients (Pts) With Muscle-Invasive Bladder Cancer (MIBC): SunRISe-4 (SR-4) Primary Analysis and Biomarker Results |
| Poster Presentations | |
|
October 18, 2025 12 – 12:45 PM CEST Abstract #3088P |
Association of Molecular Markers With Clinical Response to Gemcitabine Intravesical System in the Phase 2b SunRISe-1 Trial in Patients With BCG-Unresponsive Non-Muscle Invasive Bladder Cancer (NMIBC) With Carcinoma in Situ (CIS), With or Without Papillary Disease |
|
October 18, 2025 12 – 12:45 PM CEST Abstract #3119eP |
Real-World Use of Bacillus Calmette-Guérin (BCG) in Patients With High-Risk Non-Muscle Invasive Bladder Cancer (HR-NMIBC) |
| TAR-210 | |
| Poster Presentation | |
|
October 20, 2025 12 – 12:45 PM CEST Abstract #1766eP |
Deep Learning-Based Digital Pathology Algorithm Overcomes Tumor Content Limitations for FGFR Alteration Detection in Non-Muscle Invasive Bladder Cancer |
| Erdafitinib | |
| Poster Presentation | |
|
October 18, 2025 12 – 12:45 PM CEST Abstract #3092P |
Impact of Dose Reductions on the Efficacy of Erdafitinib (Erda) in Patients (Pts) with Advanced or Metastatic Urothelial Carcinoma (mUC): A Post-hoc Analysis of the Phase 3 THOR Study Cohort-1 Evaluating Erda versus Chemotherapy (Chemo) |
| Prostate Cancer | |
| Pasritamig | |
| Oral Presentation | |
|
October 17, 2025 2 – 3:30 PM CEST Abstract #2385MO |
Translational Analyses of T-Cell Phenotypes and Their Association With Clinical Efficacy in the First-In-Human (FIH) Trial of JNJ-78278343 (Pasritamig) in Metastatic Castration-Resistant Prostate Cancer (mCRPC) |
| Apalutamide | |
| Poster Presentations | |
|
October 18, 2025 12 – 12:45 PM CEST Abstract #2455P |
Prevalence and Severity of Hot Flashes and Their Association With Prostate-Specific Antigen (PSA) Response: Results From the Initial Treatment (Tx) Phase of LIBERTAS, a Phase 3 Study in Metastatic Hormone-Sensitive Prostate Cancer (mHSPC) |
|
October 18, 2025 12 – 12:45 PM CEST Abstract #2499P |
Overview of Therapeutic Management and Disease Progression in Patients With Localized Prostate Cancer Based on the French National Health Data System (SNDS) |
| Niraparib and Abiraterone Acetate | |
| Oral Presentation | |
|
October 17, 2025 2 – 3:30 PM CEST Abstract #LBA91 |
Patient (Pt) Reported Outcomes (PROs) From AMPLITUDE, a Randomized Placebo-Controlled Phase 3 Trial of Niraparib (NIRA) and Abiraterone Acetate (AA) Plus Prednisone (P) in Metastatic Hormone-Sensitive Prostate Cancer (mHSPC) With Homologous Recombination Repair Mutations (HRRm) |
| Poster Presentations | |
|
October 18, 2025 12 – 12:45 PM CEST Abstract #2438P |
Association of Somatic/Germline Homologous Recombination Repair (HRR) Alterations With Prostate-Specific Antigen (PSA-PFS), Radiographic (rPFS) and Second (PFS2) Progression-free Survival in Metastatic Hormone-Sensitive Prostate Cancer (mHSPC) by Disease Volume |
|
October 18, 2025 12 – 12:45 PM CEST Abstract #2515P |
Efficacy of Niraparib and Abiraterone Acetate Plus Prednisone in Metastatic Castration-Resistant Prostate Cancer (mCRPC) With Homologous Recombination Repair (HRR) Gene Alterations Identified by Tissue vs Plasma Assay in MAGNITUDE Study Final Analysis |
| Solid Tumor | |
| Early Assets | |
| Poster Presentation | |
|
October 19, 2025 12 – 12:45 PM CEST Abstract #1566P |
Phase 1 Study of Intratumoral Administration of JNJ-87704916, an Oncolytic Virus, as Monotherapy and in Combination With Cetrelimab in Advanced Solid Tumors |
About Subcutaneous Amivantamab
Subcutaneous amivantamab is an investigational fixed-dose combination of the bispecific antibody amivantamab and recombinant human hyaluronidase PH20 (rHuPH20), which is part of Halozyme’s ENHANZE® drug delivery technology. It targets EGFR and MET with immune cell-directing activity and is being developed to address tumors driven by activating and resistance EGFR mutations as well as MET alterations.
Subcutaneous amivantamab is being studied across multiple tumor types, including head and neck squamous cell carcinoma (HNSCC) and non-small cell lung cancer (NSCLC).
The legal manufacturer for subcutaneous amivantamab is Janssen Biotech, Inc.
About Gemcitabine Intravesical System
Gemcitabine intravesical system is an investigational intravesical system enabling extended release of gemcitabine into the bladder. The safety and efficacy of gemcitabine intravesical system are being evaluated in Phase 2 and Phase 3 studies in patients with non-muscle invasive bladder cancer (NMIBC) in
SunRISe-1,
SunRISe-3 and
SunRISe-5 and muscle-invasive bladder cancer (MIBC) in
SunRISe-4. SunRISe-4 is a non-registrational trial for gemcitabine intravesical system.
The legal manufacturer for gemcitabine intravesical system is Janssen Biotech, Inc.
About Niraparib and Abiraterone Acetate
Niraparib and abiraterone acetate is a combination, in the form of a dual-action tablet (DAT), of niraparib, a highly selective poly (ADP-ribose) polymerase (PARP) inhibitor, and abiraterone acetate, a CYP17 inhibitor. Niraparib and abiraterone acetate together with prednisone or prednisolone was approved in
April 2023 by the European Medicines Agency, and in
August 2023 by the U.S. FDA, for the treatment of patients with BRCA-mutated metastatic castration-resistant prostate cancer (mCRPC). Patients are selected for therapy based on an FDA-approved test for genetic alterations. Additional marketing authorization applications are under review across a number of countries globally.
Additional ongoing studies include the Phase 3 AMPLITUDE study evaluating niraparib and abiraterone acetate with prednisone or prednisolone in a biomarker-selected patient population with metastatic castration-sensitive prostate cancer (mCSPC).
In April 2016, Janssen Biotech, Inc. entered a worldwide (except Japan) collaboration and license agreement with TESARO, Inc. (acquired by GlaxoSmithKline [GSK] in 2019) for exclusive rights to niraparib in prostate cancer.
About Erdafitinib
Erdafitinib is a once-daily, oral FGFR kinase inhibitor indicated for the treatment of adult patients with locally advanced or mUC with susceptible fibroblast growth factor receptor 3 (FGFR3) genetic alterations whose disease has progressed on or after at least one line of prior systemic therapy. Erdafitinib is not recommended for the treatment of patients who are eligible for and have not received prior PD-1 or PD-(L)1 inhibitor therapy. Patients are selected for therapy based on an FDA-approved companion diagnostic for erdafitinib. Information on FDA-approved tests for the detection of FGFR genetic alterations in urothelial cancer is available at:
http://www.fda.gov/CompanionDiagnostics.
Erdafitinib received Breakthrough Therapy Designation from the U.S. FDA in 2018 and received
accelerated approval in 2019 for the treatment of adults with locally advanced or mUC that has susceptible FGFR3 or fibroblast growth factor receptor 2 (FGFR2) genetic alterations and who have progressed during or following at least one line of prior platinum-containing chemotherapy, including within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy. In addition to the FDA approval, erdafitinib received
EC approval in August 2024.
In 2008, Janssen Pharmaceutica NV entered into an exclusive worldwide license and collaboration agreement with Astex Pharmaceuticals to develop and commercialize erdafitinib.
About Apalutamide
Apalutamide is an androgen receptor inhibitor indicated for the treatment of patients with non-metastatic castration-resistant prostate cancer (nmCRPC) and for the treatment of patients with metastatic castration-sensitive prostate cancer (mCSPC). Apalutamide
received U.S. Food and Administration (FDA) approval for nmCRPC in February 2018 and
received U.S. FDA approval for mCSPC in September 2019. To date, more than 200,000 patients worldwide have been treated with apalutamide. Additional studies are ongoing in the evaluation of apalutamide for the treatment of localized high-risk or locally advanced prostate cancer including the Phase 3 ATLAS 15 (
NCT02531516) and PROTEUS (
NCT03767244) studies.
The legal manufacturer for apalutamide is Janssen Biotech, Inc.
About Johnson & Johnson
At Johnson & Johnson, we believe health is everything. Our strength in healthcare innovation empowers us to build a world where complex diseases are prevented, treated, and cured, where treatments are smarter and less invasive, and solutions are personal. Through our expertise in Innovative Medicine and MedTech, we are uniquely positioned to innovate across the full spectrum of healthcare solutions today to deliver the breakthroughs of tomorrow, and profoundly impact health for humanity. Learn more at
https://www.jnj.com/ or at
www.innovativemedicine.jnj.com. Follow us at
@JNJInnovMed.
Cautions Concerning Forward-Looking Statements
This press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding product development and the potential benefits and treatment impact of subcutaneous amivantamab, gemcitabine intravesical system, TAR-210, erdafitinib, apalutamide, cetrelimab, niraparib and abiraterone acetate and pasritamig. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to: challenges and uncertainties inherent in product research and development, including the uncertainty of clinical success and of obtaining regulatory approvals; uncertainty of commercial success; manufacturing difficulties and delays; competition, including technological advances, new products and patents attained by competitors; challenges to patents; product efficacy or safety concerns resulting in product recalls or regulatory action; changes in behavior and spending patterns of purchasers of health care products and services; changes to applicable laws and regulations, including global health care reforms; and trends toward health care cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson’s most recent Annual Report on Form 10-K, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in Johnson & Johnson’s subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. Johnson & Johnson does not undertake to update any forward-looking statement as a result of new information or future events or developments.
###
Source: Johnson & Johnson

Wondering when, where and how to watch the upcoming “Chris Hemsworth: A Road Trip to Remember” this November? We’ve got you covered. “Chris Hemsworth: A Road Trip to Remember” premieres Nov. 23 at 9/8c on National Geographic and streams next…

In the wake of the COVID-19 pandemic, there has been increasing demand for the remote delivery of health care services using information communication technologies (ICTs), including mobile phones, tablets, and computers [-]. Telehealth is defined as the use of ICTs to support and promote remote clinical health services, health education, public health, and health administration [,]. Telemedicine is a subset of telehealth that focuses on the use of ICTs for the “provision of health care services, including the exchange of medical information, diagnosis, treatment, and monitoring of patients who are not physically present with the health care provider” [].
The World Health Organization (WHO) classifies telemedicine services into one of two model types: (1) patient-to-provider, where telemedicine services are conducted between patients seeking health care services and health care providers, or (2) provider-to-provider, where telemedicine is conducted between 2 or more health care providers to provide specialized input or second opinions []. Telemedicine services may be delivered in real time (synchronously), where live interactive sessions are involved, or in a deferred mode (asynchronously), where data are stored and information is sent remotely through a remote client or patient monitoring, also known as telemonitoring. The main channels for providing telemedicine services include audio calls, SMS text messages, email, audio-video calls, smartphone or customized applications, and picture archive and communication systems [].
India (population of 1.4 billion) is home to some of the world’s earliest and largest telemedicine services []. Emerging first in the 1990s, early telemedicine services were designed and implemented by the Indian Space Research Organization (ISRO), using satellite communication to connect providers in frontline health facilities (“spokes” or peripheral hospitals) with specialists in tertiary hospitals (“hubs”) to deliver health care service remotely []. At the turn of the century, the ISRO expanded its partnership to include the Apollo private hospital network, a partnership that has evolved to include premier public sector facilities, including the All India Institute of Medical Science (AIIMS) New Delhi, the Postgraduate Institute of Medical Education and Research (PGIMER) Chandigarh, and the Sanjay Gandhi Postgraduate Institute of Medical Sciences, and additional private hospitals (Apollo, Aravind Eye Care, and Narayana Hrudayalaya) [-]. By 2015, the ISRO network had grown to include over 245 hospitals (205 district and rural hospitals and 40 superspecialty hospitals) across India [].
In the wake of COVID-19, additional telemedicine services have continued to emerge. Most notably, eSanjeevani, a national telemedicine service, was launched by the Government of India in early 2019. eSanjeevani includes both patient-to-provider and provider-to-provider telemedicine services and is currently operational in 31 states and union territories across India. Since September 2023, with the support of nearly 200,000 registered providers, eSanjeevani is reported to have served over 162 million patients through 1.08 million health and wellness centers (spokes) and 14,007 secondary or tertiary hospitals (hubs) [].
The growing digitization of health care services in India and elsewhere globally has highlighted the potential for telemedicine services to increase access to timely and appropriate care seeking, corresponding to improved health outcomes and cost savings to the individual and health system. Despite this potential, little is known about the varied typologies of telemedicine services providing in India, their design and model characteristics, scale of implementation, and the available evidence on their impact. Improved understanding of the services implemented to date, particularly at scale in India, may help to guide the efforts of future telemedicine services in other low- and middle-income countries where the disease burden is highest and the need for improved access to timely and appropriate health services is greatest.
This scoping review aims to describe the characteristics of large-scale telemedicine services initiated between 2000 and 2023 in India and to present an overview of the evidence available on these services. Study findings are anticipated to improve understanding of the vast expanse of telemedicine services offered in India and provide insights into the design, implementation, and available evidence on the impact of these telemedicine services.
This review adopted a scoping review methodology to map the breadth of telemedicine initiatives in India and generate insights into their design, implementation, and reported impact. In keeping with the objectives of scoping reviews, no formal assessment of risk of bias or methodological quality was undertaken []. The review was conducted in accordance with the framework proposed by Arksey and O’Malley [] and reported following the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) guidelines, as given in .
A comprehensive and multisource search strategy was used to identify telemedicine services in India. The primary information sources were the 3 major scientific databases, such as Embase, PubMed, and Scopus. Additionally, a Google web search was conducted to identify gray literature and programmatic reports, and the Google Play Store was searched to capture relevant mobile health applications. The reference lists of included articles were also reviewed to identify additional eligible studies. This combined approach ensured that both published evidence and real-world implementations not indexed in traditional databases were included. Detailed search strategies for each source are provided in .
Telemedicine was defined as including (1) a health care expert (doctor, nurse, physical therapist, or nutritionist) who makes (2) decisions tailored to a specific patient profile, through (3) a digital solution, including phone, computer, or tablet. “Formal” telemedicine services were defined as digital communication sanctioned by the organization and used according to a protocol. Telemedicine services were further categorized based on the reported scale of their implementation and considered to be moderate to large in scale if they met one or more of the following criteria: (1) a minimum of 1000 app downloads or patients reached, catered to, or consultations conducted, and (2) implemented in >1 hospital or geographical location.
Included telemedicine services were restricted to those that included humans, were published in the English language between January 1, 2010, and July 4, 2023, and pertain to services in India. Studies were excluded if they (1) were 1-way direct-to-beneficiary applications that provide information only, (2) relied on informal technology use by providers, such as personal telephone calls or patient contact solely on publicly available chat applications (eg, WhatsApp), (3) focused on data capture, workflow support applications, clinical decision-making algorithms, or job aids, including those that use artificial intelligence to render a diagnosis or are used by providers to screen patients in the course of home visits, (4) pertained to e-training or e-mentoring services, or (5) self-monitoring services, including those involving artificial intelligence or chatbots. Articles focusing solely on the technical specification of internet connectivity, book reviews, and conference proceedings were also excluded.
Once identified, articles were imported to Covidence (Veritas Health Innovation Ltd), and the process of abstract screening was initiated using 2 independent reviewers and a third person to resolve conflicts. Full-text articles were screened by 2 independent reviewers and a third person to resolve conflicts. Data from the full-text articles were extracted into Microsoft Excel. To ensure alignment across reviewers with the data extraction, weekly meetings were held across the study team. Senior investigators additionally conducted spot checks of articles to review their classification and the data extracted.
summarizes the extraction domains across three broad categories: (1) model type, (2) model characteristics, and (3) reach and impact. The model type includes the health delivery sector (public, private, or public-private partnership [PPP]) and the WHO classification type (provider-to-provider, patient-to-provider, or both). Model characteristics include key stakeholders, services provided, timing of delivery, service delivery channel, licensing provisions, monitoring, and learning and evaluation activities. Reach and impact include details on the scale of implementation and evidence on effectiveness where reported.
Given that this is a scoping review and not a systematic evidence synthesis or meta-analysis, we did not assess the quality of evidence reported in individual articles. Rather, the goal of this scoping review was to identify the full range of telemedicine services, including those for which peer-reviewed articles have not been published. Findings from peer-reviewed articles on the effectiveness of telemedicine sought to provide a broad overview of the landscape of evidence across disparate types of research and areas of inquiry.
Efforts to synthesize details on the model characteristics of the telemedicine service sought to follow the framework in . Efforts to collate evidence on effectiveness drew from the evaluation categories depicted in WHO’s guidance on the monitoring and evaluation of digital health interventions [].
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram in provides a summary of the screening process. From the peer-reviewed article databases, 2366 articles were identified, and after the exclusion of 716 duplicates, the abstracts from 1650 articles were screened for eligibility. Of these, 1205 were excluded, and 445 articles were deemed eligible for full-text review. A total of 151 studies were included for the full-text review and data extraction, including 2 articles identified from the references of other articles.
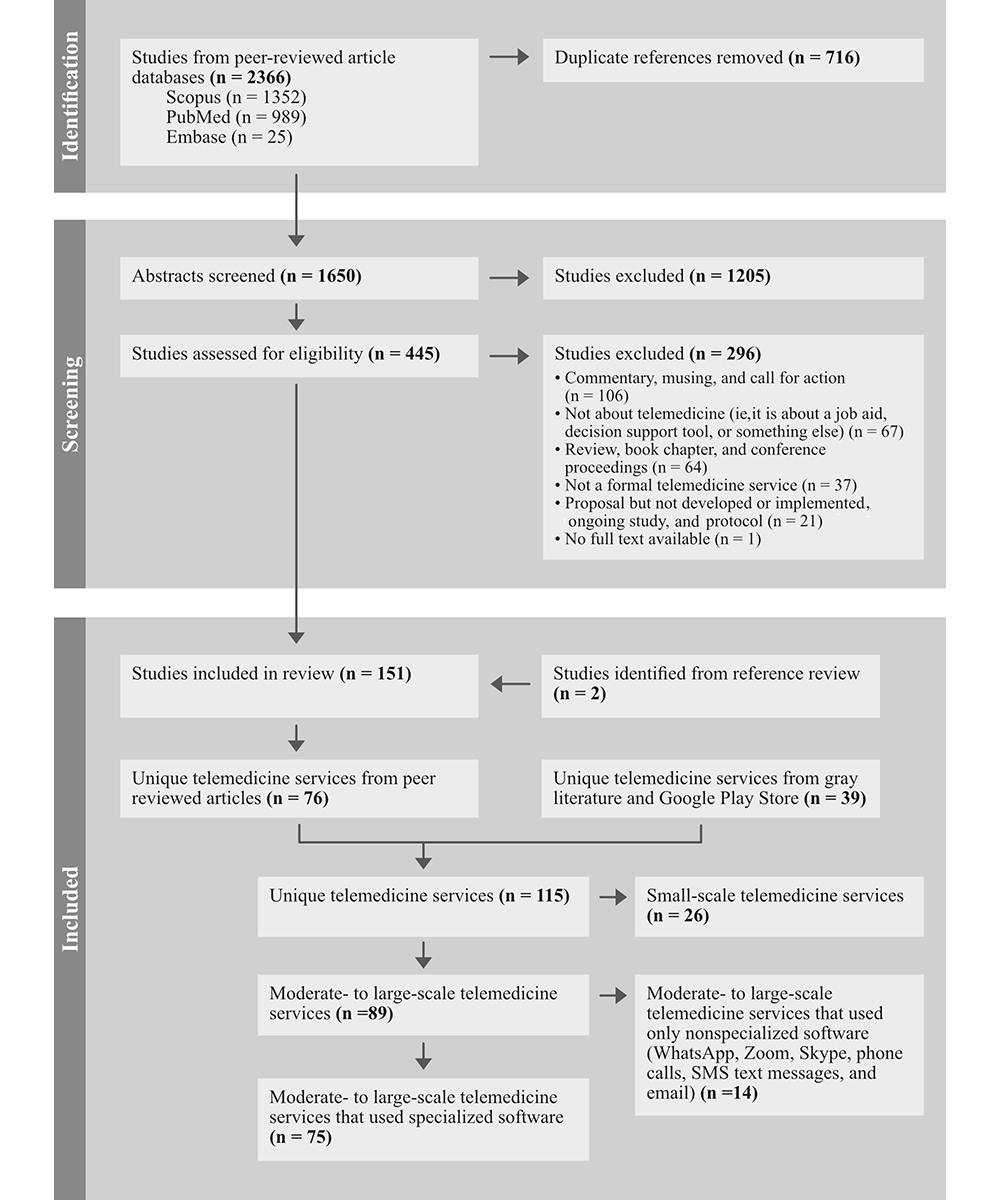
To identify unique telemedicine services, we categorized peer-reviewed articles by name and additionally reviewed the gray literature and Google Play Store. A total of 115 unique telemedicine services were identified (76 from the peer-reviewed literature and 39 from gray literature and the Google Play Store). Unique telemedicine services were further classified based on (1) scale and (2) reported use of specialized software. Among the 115 unique services, 89 (77%) were classified as being moderate to large in scale, and 26 (23%) were small. Large scale is operationalized as those services that met one or more of the following criteria: (1) a minimum of 1000 downloads, patients, or consultations, and (2) implemented in >1 hospital or geographical location. Among the 89 moderate- to large-scale services, 75 used specialized software and 14 used nonspecialized software, such as WhatsApp. The tables and figures that follow present extracted data for the unique moderate- to large-scale services that reported using specialized software (n=75).
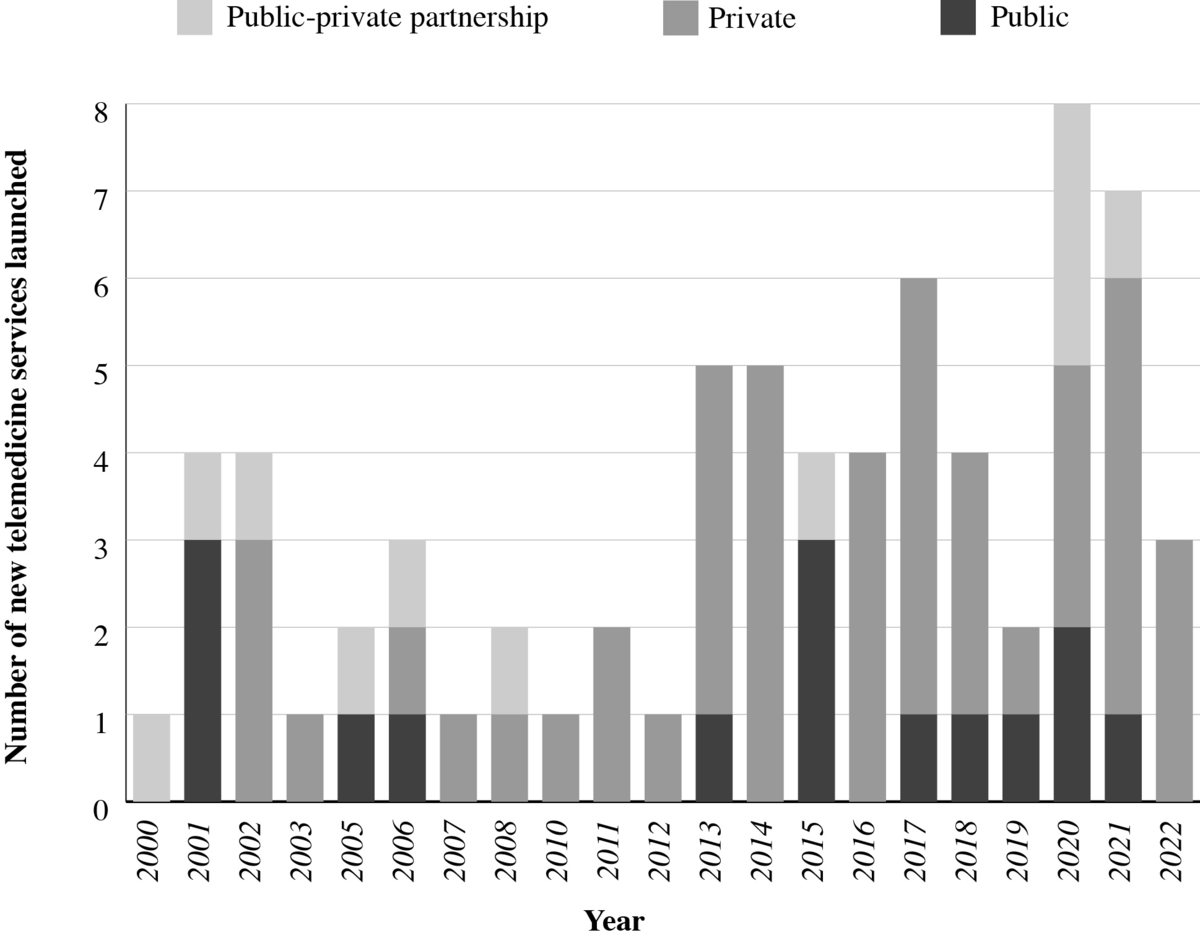
shows the distribution of moderate- to large-scale unique telemedicine services using specialized software initiated or launched between 2000 and 2023. On average, 3 new telemedicine services were initiated annually from 2000 to 2019, and the growth of new services occurred predominantly in the private sector. The start of the COVID-19 pandemic in 2020 corresponds to an increase in the number of new telemedicine services.
presents summary characteristics of moderate- to large-scale telemedicine services using specialized software in India. Out of 75 services, 64% (48/75) were delivered by the private sector, while 19% (14/75) were public sector and 17% (13/75) were PPP. Nearly half (37/75) of the services were provided through a patient-to-provider model, 24% (18/75) provider-to-provider, and one-third (20/75) using both patient-to-provider and provider-to-provider models. Services were provided in real time (synchronous) for 69% (52/75), while 28% (21/75) of services delivered both synchronous and asynchronous services, and 3% (2/75) delivered only asynchronously. While most services (52/75, 69%) offered multispecialty care covering 2 or more health domains or conditions, one-third (23/75) focused on condition-specific care (eg, ophthalmology or mental health). All services in both public (14/75, 19%) and PPP (13/75, 17%) sectors were provided with limited (nominal charges for outpatient registration) to no fees. In the case of private sector services (n=48), service fees ranged from US $2.40 (INR 200) to US $7.21 (INR 600) per service, and for some services, monthly subscription fees ranging from US $18 to US $32 (INR 1500-3000) were charged depending upon the services beneficiaries subscribed to. For some private sector telemedicine services, beneficiary charges occurred indirectly through the purchasing of insurance and other employee wellness schemes.
| Telemedicine service characteristics | Values, n (%) | ||
| Health delivery sector | |||
| Public | 14 (19) | ||
| Private | 48 (64) | ||
| Public-private partnership | 13 (17) | ||
| Model type per WHOa classification | |||
| Provider-to-provider | 18 (24) | ||
| Patient-to-provider | 37 (49) | ||
| Both | 20 (27) | ||
| Timing of delivery | |||
| Synchronous or real-time | 52 (69) | ||
| Asynchronous | 2 (3) | ||
| Both | 21 (28) | ||
| Health domain or condition | |||
| Multispecialty | 52 (69) | ||
| Condition specific (eg, ophthalmology or mental health) | 23 (31) | ||
aWHO: World Health Organization.
outlines the details of key stakeholders engaged in the implementation of moderate- to large-scale telemedicine services. The earliest telemedicine services involving the public sector were initiated by the ISRO with support of other government bodies, including the Department of Information Technology, Ministry of External Affairs, Ministry of Health and Family Welfare, and the state governments []. More recent telemedicine services have been led by the Ministry of Health and Family Welfare at the national level, in coordination with state governments for implementation (14/75, 19%). The public sector included both models of service delivery, that is, patient-to-provider (5/14) and provider-to-provider (2/14). In contrast, the majority of private sector services (48/75, 64%) were patient-to-provider (30/48), through one of two categories: (1) networks of hospitals (16/48, 33%) or (2) technology service providers (32/48, 67%) who created technology solutions. The latter included business-to-business for third-party health care providers (n=8) and business-to-consumer technology solutions for patients and providers (n=24).
| Health delivery sector | Public (n=14), n (%) | PPPa (n=13), n (%) | Private (n=48), n (%) | ||
| Model type | |||||
| Provider-to-provider | 2 (14) | 5 (38) | 11 (23) | ||
| Patient-to-provider | 5 (36) | 2 (16) | 30 (62) | ||
| Both | 7 (50) | 6 (46) | 7 (15) | ||
| Implementing organization | |||||
| Networks of hospitals | N/Ab | N/A | 16 (33) | ||
| Technology service providers (B2Bc) | N/A | N/A | 8 (17) | ||
| Technology service providers (B2Cd) | N/A | N/A | 24 (50) | ||
| Clinical and service providers | |||||
| MBBS doctors or higher-level specialists | 14 (100) | 13 (100) | 48 (100) | ||
| Dentists | 0 (0) | 0 (0) | 11 (23) | ||
| AYUSHe practitioner | 1(7) | 1 (8) | 7 (15) | ||
| Allied health services | 4 (29) | 4 (31) | 11 (23) | ||
| Patients (age group) | |||||
| All age groups | 13 (93) | 13 (100) | 48 (100) | ||
| Specific (pediatric) | 1 (7) | 0 (0) | 0 (0) | ||
aPPP: public-private partnership.
bN/A: not applicable.
cB2B: business to business.
dB2C: business to consumer.
eAYUSH: Ayurveda, Yoga and Naturopathy, Unani, Siddha and Homeopathy.
All 75 (100%) telemedicine services had MBBS doctors or higher-level specialists as clinical providers, while 25% (19/75) included access to allied health services, 13% (10/75) to dentists, and 12% (9/75) to AYUSH (Ayurveda, Yoga and Naturopathy, Unani, Siddha, and Homeopathy—the 6 Indian systems of medicine) practitioners. Among beneficiaries, only 1 telemedicine limited services to pediatric patients, while the remainder (74/75, 99%) catered to patients of all age groups. Technology, monitoring and evaluation, and funding of the services were either not reported or limited. Detailed description of key stakeholders for each telemedicine services is provided in Multimedia appendix 3.
The scale of implementation for the moderate- to large-scale telemedicine services using specialized software is summarized in . Services reported their scale of implementation using a wide range of parameters, and no common standard has yet been developed. Thus, we gather information on scale across the following parameters based on available information: (1) geographic areas (state and districts) of implementation, (2) number of registered providers, (3) number of spokes and hubs, (4) number of patients served or treated, (5) number of consultations (overall or daily) or prescriptions, and (6) number of downloads on the Google Play Store. Information for at least 1 scale parameter was reported in 75 telemedicine services.
Among public sector services, as of July 19, 2023, eSanjeevani reported the largest number of registered providers (n=185,100) and health facilities (>100,000 primary health clinics and >13,000 secondary and tertiary hospitals) and is operational across 31 states and union territories across India. The total number of patients served was reported to exceed 138 million, and over 10 million consultations were carried out from November 2019 to July 2023. Among PPP models identified, Apollo telehealth services reported providing services in over 350,000 telemedicine centers, Apollo clinics, and common service centers, and 73 Apollo hospitals across 14 states in India. From 2000 to 2023, Apollo services reportedly reached over 13 million patients and delivered over 16 million teleconsultations. Among private sector–only models (n=48), 17% (8/48) reported having conducted over 1 million consultations. Practo, a private sector service that launched in 2008, provides services through over 0.1 million doctor partners. provides a brief overview of the 5 largest telemedicine services in India that use specialized software.
eSanjeevani
Indian Space Research Organization (ISRO)
National Telemedicine Network
Apollo group of hospitals
Aravind Eye Care (teleophthalmology)
Practo
Over a quarter (21/75, 28%) of the moderate- to large-scale services that used specialized software were being implemented in 1 state. The remaining services are implemented in multiple states—17% (13/75) in fewer than 10 states and 12% (9/75) in 10 or more states—or did not report any geographical location (4/75, 5%). All telemedicine service applications identified from the Google Play Store (28/75, 37%) were accessible in all states across India. However, for some of these (7/28, 25%), accessibility within states was limited to either major cities or certain parts of the state.
Beyond the distribution of telemedicine services across and within states, information on the number of “registered” or “active” providers was reported for only 20% (15/75) of services. For those services that reported this information, the number of active or registered providers ranged from 5 to 0.5 million, with 33% (5/15) reporting 100,000 or more providers. Telemedicine reach in terms of the number of patients served, treated, or “lives saved,” or the number of consultations or prescriptions provided, was reported for 61% (46/75) of services. For the 46 services that reported reach, 36 (78%) served less than 1 million patients or provided consultations, 8 services had between 10 and 20 million, and only 2 services reported more than >100 million patients served (eSanjeevani and Practo). Among the telemedicine applications in the Google Play Store, 61% (17/28) had fewer than 1 million downloads, and 39% (11/28) had more than 1 million, ranging up to >100 million downloads (4 telemedicine services).
We examined peer-reviewed research articles for evidence on the effectiveness of the 75 moderate- to large-scale telemedicine services. We considered an article to include evidence of effectiveness if it provided information on processes, outcomes, or impact. This included but was not limited to studies on reach, quality of care, economic evaluation, or provider or patient perceptions of the service. Details on the evaluation were extracted, including study design, methods, and findings ().
| Evidence of effectiveness | Moderate- to- large-scale telemedicine services reporting effectiveness (n=75), n (%) | Articles reporting on effectiveness (n=84), n (%) | ||||||
| Inputs | ||||||||
| Technological readiness | 20 (27) | 34 (40) | ||||||
| Patient readiness | 6 (8) | 9 (11) | ||||||
| Provider readiness | 15 (20) | 28 (33) | ||||||
| Structural readiness | 14 (19) | 17 (20) | ||||||
| Processes | ||||||||
| Technical care | 14 (19) | 20 (24) | ||||||
| Interpersonal and respectful care | 11 (15) | 21 (25) | ||||||
| Technological performance | 9 (12) | 11 (13) | ||||||
| Patient-provider engagement with technology | 5 (7) | 8 (10) | ||||||
| Outcomes | ||||||||
| Experience of care | 17 (23) | 36 (43) | ||||||
| Costs, time savings | 14 (19) | 25 (30) | ||||||
| Health outcomes | 24 (32) | 52 (62) | ||||||
| Provider capacity (at the spoke level) | 3 (4) | 5 (6) | ||||||
| Equity | 3 (4) | 4 (5) | ||||||
| Gender inclusion | 2 (3) | 2 (2) | ||||||
| Economic evaluation | ||||||||
| Cost-effectiveness or cost-utility | 6 (8) | 7 (8) | ||||||
| Cost outcome (telemedicine service costing analysis) | 4 (5) | 4 (5) | ||||||
| Data sources | ||||||||
| System-generated data analysis | 7 (9) | 8 (10) | ||||||
| Structured survey (patients and providers) | 19 (25) | 39 (46) | ||||||
| Qualitative methods: in-depth interviews and focus group discussions | 6 (8) | 6 (7) | ||||||
| Medical record review | 23 (31) | 45 (54) | ||||||
| Clinical observation | 3 (4) | 3 (4) | ||||||
| Vignettes | 1 (1) | 1 (1) | ||||||
| Study design | ||||||||
| Descriptive | ||||||||
| Surveillance | 0 (0) | 0 (0) | ||||||
| Ecological correlation | 0 (0) | 0 (0) | ||||||
| Cross-sectional (prevalence) | 22 (29) | 50 (60) | ||||||
| Case report | 6 (8) | 6 (7) | ||||||
| Qualitative | 6 (8) | 6 (7) | ||||||
| Analytic | ||||||||
| Experimental with randomization | 0 (0) | 0 (0) | ||||||
| Quasi-experimental | 3 (7) | 8 (11) | ||||||
| Observational: cohort | 6 (8) | 7 (7) | ||||||
| Observational: cross-sectional | 8 (12) | 10 (13) | ||||||
| Observational: case-control | 1 (1) | 1 (1) | ||||||
Evidence on effectiveness was available for 43% (32/75) of the services, reported across 84 articles. PGIMER Chandigarh’s telemedicine service was the most studied in terms of service effectiveness, with 10 articles published. The National Institute of Mental Health and Neuro Sciences Bengaluru telemedicine service was a close second with 7 articles, while 14 of the 32 services with evidence on effectiveness had just 1 published article covering this topic (). See for details by telemedicine service and article.
Given the large number of studies across a range of designs, we focus here on a synthesis of findings from the analytic research on health outcomes. Evidence of the effectiveness of telemedicine on health outcomes (which includes impact on patient access to care, diagnosis, and morbidity) was reported in 52 of 84 articles for 24 of 75 telemedicine service (), of which 18 articles for 11 services provided analytic evidence (see ). The remaining articles reported solely descriptive findings.
Within the set of 18 analytic articles on health outcomes, none were randomized controlled trials. A total of 7 were quasi-experimental studies on 3 services: the Pediatric HIV Telemedicine Initiative in Maharashtra [-], the World Health Partners’ Sky Program in Uttar Pradesh and Bihar [-], and Aravind Eye in Tamil Nadu []. The clinical management of children living with HIV in centers linked with the Pediatric HIV Telemedicine Initiative was better compared to nonlinked centers [-,]. Fewer patients were lost to follow-up at the centers with the Pediatric HIV Telemedicine Initiative, but there was no difference in the proportion of patients with delayed treatment once the telemedicine service reached its later phase of implementation []. The World Health Partners’ Sky Program showed no improvement in the quality and coverage of maternal health services at the population level [], no improvement in treatment for childhood diarrhea and pneumonia, nor reduced prevalence of these diseases before and after implementation [], nor did it change provider knowledge []. Opening an Aravind Eye telemedicine center staffed by mid-level (nonphysician) providers led to a significant increase in overall network visit rates and rates of eyeglasses prescriptions for the population living within 10 km of the new center [].
The 11 remaining analytical studies on health outcomes consisted of 5 observational cohort studies on 5 services [-], 5 observational cross-sectional studies on 5 services [-], and 1 observational case-control study []. Among the observational cohort studies, 2 found that telemedicine was associated with patient improvements; patient mental health scores significantly improved post telepsychiatry treatment in Goa [], and patients showed a significant reduction in hemoglobin A1c (HbA1c) test result from baseline to follow-up while receiving telemedicine support through the Diabetes Tele Management System at Jothydev’s Diabetes and Research Center in Kerala []. One found no significant difference in functional assessment of “overdentures” (dentures anchored to teeth or modified roots) fabricated by newly graduated students who were guided remotely through provider-to-provider telemedicine versus guided in person at a university teaching hospital []. Furthermore, 2 reported that telescreening for retinopathy of prematurity was suitable to assess incidence over time [,].
The observational cross-sectional studies found that the use of telemedicine for diagnosis was equal to in-person models or brought added benefit. The 2 found comparable levels of diagnosis between telemedicine and in-person care: school hearing tests conducted by doctors through a remote audiometer, Distortion Product Otoacoustic Emissions system, and video-otoscope compared to doctors in person [], and diabetic retinopathy screening conducted by doctors through Dr Mohan’s Diabetes Specialties Center’s teleophthalmology compared to doctors in person []. Comparing the diagnosis of head and neck tumors made in person by clinicians at Amrita Institute of Medical Sciences, Kochi, versus remotely by colleagues in the United States found high concurrence, low differential diagnosis, and some additional diagnoses []. Sankara Nethralaya’s telescreening model diagnosed a higher prevalence of diabetic retinopathy compared to the in-person ophthalmologist-based screening camp model and found more sight-threatening retinopathies []. Finally, a higher portion of children went for diagnosis referral to telediagnostic auditory brainstem response (ABR) compared to in-person ABR (97% taken to telediagnostic ABR appointment vs 80% taken to ABR appointment) [].
The observational case-control study compared virtual diabetes care using the Diahome app to hospital outpatient service use and found that app users had a greater reduction in HbA1c (but higher triglycerides throughout) []. The remaining studies on health outcomes were descriptive, describing patient outcomes without a comparator.
Data on patient or provider costs for telemedicine services were reported in 21 studies. The predominant means of measuring costs was through structured surveys, which asked respondents about perceived savings of time and money [-], future willingness to pay for teleconsultation costs [], or actual costs incurred. Regarding the latter, in a limited number of studies, a broad range of cost-related outcomes were assessed, including distance traveled to seek care [,-], food and overnight charges [], consultation and clinical costs [,,,,-], waiting time [], and reported lost workdays [-]. These were used to collectively estimate costs and cost savings attributed to telemedicine services from a range of perspectives.
Costing analyses, which presented data on the costs of a single telemedicine service, were reported in 4 articles. These studies sought to present evidence on the telemedicine costs needed to establish the service [,,]. Data on the cost-effectiveness and cost-utility of telemedicine services were reported in 8 articles for 9 moderate- to large-scale services. The methods, including the perspective from which costs and effects were derived, the primary and secondary data sources, the analytic time horizon used, and sensitivity analyses conducted, varied widely across studies, which impeded efforts to draw cross-cutting syntheses of findings.
Scoping review findings led to the identification of 2368 articles from which 151 studies and 115 unique telemedicine services were identified and further categorized based on their scale of implementation and use of specialized software. Among moderate- to large-scale services (n=89), 75 used specialized software in isolation or augmented with telephone calls, WhatsApp, Zoom, and other nonspecialized software. Of these 75 services, 64% (48/75) were in the private sector, and the rest were either public or in partnership with private actors. The patient-to-provider model was the model that nearly half (37/75) of the telemedicine used to deliver their services. Telemedicine services were provided in real time (synchronous) for 69% (52/75), and 28% (21/75) delivered both synchronous and asynchronous services. Evidence was available for 43% (32/75) of the services.
Efforts to differentiate telemedicine services based on their scale of implementation and use of software sought to narrow emphasis in a crowded space, removing the “‘noise” of services established ad hoc within a limited geography or health setting, or without the software arguably needed to scale or accommodate the structural and procedural access controls for handling sensitive personal health data. Use of nonspecialized software may stem from user preferences, wherein providers and patients are more comfortable using existing software already on their phones, or may be driven by specialized software shortcomings. In situations where the specialized software crashes or has limited functions (ie, is only suitable for booking appointments or cannot be used for bidirectional sharing of photos and documents), patients and providers may shift to nonspecialized software. This ongoing use of nonspecialized software has enabled telemedicine services to scale but may have some drawbacks. Using specialized software allows each consultation to be integrated with electronic medical records, enabling backend data on call duration and other parameters to be tracked. In cases where the use of nonspecialized software persists, facilities may want to ensure that providers use telemedicine only on official phones, thereby protecting patient data and ensuring separation of work and personal life for providers.
Data on the typologies of telemedicine models sought to distinguish between provider-to-provider, patient-to-patient, and hybrid models. The fact that public sector services used both models suggests that telemedicine is being operationalized as a health system–strengthening intervention in addition to improving patient access to services by the government. By comparison, in the private sector, the implementation of telemedicine services seemed to focus on the use of telemedicine to expand accessibility and reach.
We found that many departments in large hospitals such as AIIMS New Delhi, Jawaharlal Institute of Postgraduate Medical Education and Research, PGIMER Chandigarh, and Apollo used the hospital-wide telemedicine services in different ways, according to their department’s needs. For instance, at AIIMS New Delhi, we found that 6 departments were using telemedicine and that some had used it for over 6000 patients (eg, pediatrics) [], while others had used it for just 314 (eg, oncology palliative medicine) []. Some reported using only special software, while others reported augmenting this software with WhatsApp or telephone calls.
Study findings on the evolution of telemedicine services in India cement India’s place as a global leader in the use of technology for health. In other low-resource settings, the field is characterized by fragmentation and driven by private sector and nongovernment organization–led models with limited scale and reach. Nigeria is home to several telemedicine initiatives, including the World Telehealth Initiative [], which aims to expand health care access through a clinical mentorship service in Opoji, Nigeria, and Hudibia (established in 2013), which is an application-based solution that allows users to search and see doctors through videoconferencing or to book a face-to-face appointment []. In Ghana, a recent review of telemedicine services [] identified a small number of services, including Bima, which uses a direct-to-patient model to provide health advice and succinct health education to Ghanaians. Elsewhere regionally, HelloDoctor in South Africa [] and Babyl Rwanda [] are private sector models that aim to bolster access to medical doctors and nurses as well as a range of clinical and laboratory services directly to the phones of beneficiaries. Data on the uptake of these services are limited.
The wide breadth and variety of telemedicine services, including public and private sector–led and types of telemedicine models (patient-to-provider or provider-to-provider), render comparisons challenging. However, India is unique for a number of reasons. From a supply-side perspective, the government is investing heavily in national telemedicine services via eSanjeevani (established in 2019), which includes both a patient-to-provider and a provider-to-provider model. While there is limited evidence on the reach and impact of both eSanjeevani models, the service has scaled widely [] with support from Ayushman Bharat Digital Mission and other government initiatives. From a demand side, less than half of women in India report having access to a mobile phone that they themselves can use []. Further barriers to women’s use of technology [] are likely to limit the reach and use of patient-to-provider telemedicine services in India, particularly in rural areas. Emerging data on the limited uptake of eSanjeevani’s patient-to-provider model reinforces these challenges.
The large volume of studies has necessitated that we narrow our focus to unique telemedicine services that are moderate- to large-scale and report using specialized software. The central challenge in reporting the scale was that few services publicly list information on the scale of implementation, including the number of active providers and consultations completed. Those that do have listings have varied definitions for key constructs. For example, unique consultations versus the number of patients treated, and active versus registered providers. While we extracted information on the reported evidence generation, given the volume and variety of methodological approaches undertaken, we have not taken into account the quality of evidence reporting.
The widespread proliferation of telemedicine services in India has much potential to improve access to and continuity of timely and appropriate care seeking for health. However, our findings highlight significant limitations in evidence generation and reporting. Future research is needed to bolster independent evidence gathering on the impact that telemedicine services may have in bolstering equitable access to timely, continuous health services of equivalent or better quality than face-to-face services. Further data on costs to beneficiaries, including any cost savings, as well as assurances that remote service delivery does not compromise beneficiary experiences, are needed.
The authors would like to thank the Gates Foundation’s India Country Office for providing funding support and important insights used to optimize the presentation of findings. We thank Dan Harder of the Creativity Club UK for his efforts to improve the figures presented. We also extend our thanks to Jai Mendiratta for his support in facilitating the work.
Data curation: OU (lead), AL (equal), KS (equal), AS (supporting), AK (supporting), DM (supporting)
Writing—original draft: OU (lead), AL (equal), KS (equal), DM (supporting), AS (supporting), AK (supporting)
All authors have read and approved the final version of the manuscript.
None declared.
Edited by T Leung, G Eysenbach; submitted 09.Jul.2024; peer-reviewed by A Orchanian-Cheff, A Venkataraman, M Sadiq; comments to author 08.Nov.2024; revised version received 05.May.2025; accepted 28.Aug.2025; published 15.Oct.2025.
©Osama Ummer, Anjora Sarangi, Arjun Khanna, Diwakar Mohan, Kerry Scott, Amnesty LeFevre. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 15.Oct.2025.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research (ISSN 1438-8871), is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.

On the gut health side of TikTok there’s a genre of videos where people jokingly celebrate having a…

A

Razer is updating its lineup of creator-focused webcams with two new models, the Razer Kiyo V2 and Razer Kiyo V2 X. The webcams offer new AI-powered features and 4K streaming at a more affordable price than the company’s Kiyo Pro webcams.
The $100…

Egypt’s dominance extended to the doubles events, where the nation swept all three titles – mixed doubles, women’s doubles, and men’s doubles.
In the mixed doubles final, defending champions Youssef…