Apple’s AirTags have become one of my essentials while traveling, namely because they let me check whether my luggage is in the right place instead of panicking at the baggage claim. And right now, you can get a four-pack of AirTags at Amazon…
Blog
-

46.5% Profit Growth Reinforces Value Narrative
Popular (BPOP) posted a 46.5% earnings growth over the past year, representing a sharp turnaround from its longer-term average decline of 3.4% per year. With net profit margins rising to 27% compared to 20.2% last year, investors saw a meaningful boost in profitability and operational efficiency. Looking forward, the setup includes forecasts for continued growth, attractive valuation multiples compared to peers, and an improving value proposition for shareholders.
See our full analysis for Popular.
The next section puts these headline numbers in context by measuring them against the main market narratives that shape how investors view Popular’s future potential.
See what the community is saying about Popular
NasdaqGS:BPOP Earnings & Revenue History as at Oct 2025 -
Net profit margins currently stand at 27%, up from 20.2% the prior year. This reflects a significant step up in operational efficiency compared to recent history; however, margins have not yet reached the analysts’ long-term forecasted average of 24.7% in three years.
-
Analysts’ consensus view sees current margins helping Popular outperform regional peers in near-term profitability,
-
but the forecasted slip to 24.7% suggests cost or competitive pressures could gradually erode this advantage,
-
even as ongoing investments in digital platforms and infrastructure modernization are seen as key to defending those margins over the longer term.
-
Consensus narrative underscores how Popular’s stronger margins give the company breathing room as it ramps up digital investments and positions for a more competitive landscape.
📊 Read the full Popular Consensus Narrative.-
Popular is trading at just 9.8x price-to-earnings, undercutting both US banks peers (10.9x) and the broader US Banks industry (11.2x). Analysts cite this as a sign the market may not be fully crediting recent margin and profit improvements.
-
Analysts’ consensus view flags the low PE as a value opportunity,
-
especially when weighed against the 9.5% forecasted revenue growth that runs nearly in line with the US average of 10%,
-
though they caution that any stalling in modernization, slow digital adoption or regional risks could keep the stock discounted relative to national banks.
-
-
With analysts expecting the number of shares outstanding to fall by 5.22% per year over the next three years, future per-share earnings growth could be amplified relative to headline net income expansion.
-
Analysts’ consensus view calls out prudent share repurchases and solid value metrics as supporting a “total return” case,
-
since the share count reduction and current attractive valuation set a strong platform for outperformance,
-
but they remain alert to regional risks, like Puerto Rico concentration and rising deposit competition, that could complicate this scenario.
-
Continue Reading
-
-

Novartis showcases significant immunology advancements in ACR congress with new data in complex autoimmune diseases
- Late-breaking positive Phase III data from ianalumab NEPTUNUS-1 and NEPTUNUS-2 trials in Sjögren’s disease to be presented
- Biomarker data informing use of investigational CAR-T cell therapy rapcabtagene autoleucel (YTB323) in systemic lupus erythematosus also to be presented
- Data underscore Novartis commitment to advance innovative medicines for complex, difficult-to-treat autoimmune diseases with high unmet need
- Novartis to hold virtual investor event following ACR highlighting immunology pipeline progress
Basel, October 25, 2025 – Novartis announced today plans to present data from 27 company- or investigator-sponsored abstracts across its Immunology portfolio and pipeline at the 2025 American College of Rheumatology (ACR) Convergence. Data to be presented include late-breaking pivotal Phase III results from the replicate NEPTUNUS-1 and NEPTUNUS-2 trials evaluating ianalumab in Sjögren’s disease1. New biomarker data from an ongoing Phase 1/2 study of rapcabtagene autoleucel in severe refractory systemic lupus erythematosus will also be presented, along with Cosentyx data in multiple rheumatology indications2,3.
“Our data at this year’s ACR demonstrate that Novartis is at the forefront of scientific innovation and is developing medicines for some of the most challenging autoimmune diseases, such as Sjögren’s,” said Angelika Jahreis, Global Head, Development, Immunology, Novartis. “Autoimmune diseases are often devastating and life-limiting. We are committed to developing new therapies with the potential to transform the standard of care for the millions who continue to suffer from rheumatic diseases.”
Ianalumab is an investigational medicine that has the potential to become the first targeted therapy for Sjögren’s disease, an area of high unmet need with no FDA-approved treatments4,5. Sjögren’s disease affects millions of people globally and is the second most prevalent rheumatic disease6.
Additional presentations include data for rapcabtagene autoleucel, a novel one-time investigational CAR-T cell therapy being evaluated across several refractory autoimmune disease for its potential to induce an immune reset7-9. Further presentations will feature real-world data on Cosentyx® (secukinumab) in psoriatic arthritis, and new insights into the dual mode of action of ianalumab.
Investor call on Novartis Immunology pipeline
Following the conclusion of ACR, Novartis will host a conference call for investors to provide updates on the company’s Immunology pipeline on Thursday, October 30, 2025, at 11:30 a.m. ET. Details can be found here.Key abstracts accepted by ACR include:
Molecule/disease state Abstract title Abstract number/ presentation details Ianalumab Sjögren’s disease Ianalumab demonstrates significant reduction in disease activity in patients with Sjögren’s Disease: Efficacy and safety results from two global Phase 3, randomized, placebo-controlled double-blind studies (NEPTUNUS-1 and NEPTUNUS-2) Abstract #LB24
Oral presentation
Oct. 29, 9:15 am – 9:30 am CSTSjögren’s disease Evaluation of the dual mode of action of Ianalumab (VAY736) in the circulation and salivary gland tissue of patients with Sjögren’s Disease: Results from a Phase 2 mechanistic study Abstract #2296
Poster presentation
Oct. 28, 10:30 am – 12:30 pm CSTSjögren’s disease Ianalumab’s dual mode of action: targeting B cells through enhanced B cell depletion and blockade of B cell activating factor receptor signaling Abstract #0903
Poster presentation
Oct. 27, 10:30 am – 12:30 pm CSTSystemic lupus erythematosus Achieving sustained lupus low disease activity state and remission with ianalumab (VAY736) in patients with systemic lupus erythematosus: A post hoc analysis from a phase II study Abstract #0801
Oral presentation
Oct. 26, 1:00 pm – 1:15pm CSTRapcabtagene autoleucel Systemic lupus erythematosus Biomarker data from an open-label, Phase 1/2 Study for YTB323 (Rapcabtagene Autoleucel, a rapidly manufactured CD19 CAR-T therapy) suggest reset of the B Cell compartment in severe refractory SLE Abstract #2696
Oral Presentation
Oct. 29, 12:15pm – 12:30 pm CSTCosentyx (secukinumab) Psoriatic arthritis Comparison of incidence of psoriatic arthritis in patients with psoriasis treated with interleukin-17 inhibitors vs interleukin-23 inhibitors, interleukin-12/23 inhibitors, and tumor necrosis factor inhibitors in real-world practice: a retrospective study Abstract #2689
Oct. 29, 12:15pm – 12:30 pm CSTAbout Novartis Immunology
At Novartis, we’re advancing bold science for autoimmune diseases, where meaningful therapeutic progress has long stalled.With a growing legacy of first-in-class innovation across Rheumatology, Dermatology and Allergy, and a diverse industry-leading pipeline, we’re committed to shaping what’s next in Immunology. From small molecules to biologics and CAR-T cell therapy, our innovation is powered by cutting-edge science, focused on where we can have the greatest impact on patient outcomes and supported by strong collaboration across the healthcare ecosystem.
We’re not just treating autoimmune diseases. We’re reimagining medicine, together.
Product information
For full prescribing information, including approved indications and important safety information about marketed products, please visit https://www.novartis.com/about/productsDisclaimer
This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements can generally be identified by words such as “potential,” “can,” “will,” “plan,” “may,” “could,” “would,” “expect,” “anticipate,” “look forward,” “believe,” “committed,” “investigational,” “pipeline,” “launch,” or similar terms, or by express or implied discussions regarding potential marketing approvals, new indications or labeling for the investigational or approved products described in this press release, or regarding potential future revenues from such products. You should not place undue reliance on these statements. Such forward-looking statements are based on our current beliefs and expectations regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that the investigational or approved products described in this press release will be submitted or approved for sale or for any additional indications or labeling in any market, or at any particular time. Nor can there be any guarantee that such products will be commercially successful in the future. In particular, our expectations regarding such products could be affected by, among other things, the uncertainties inherent in research and development, including clinical trial results and additional analysis of existing clinical data; regulatory actions or delays or government regulation generally; global trends toward health care cost containment, including government, payor and general public pricing and reimbursement pressures and requirements for increased pricing transparency; our ability to obtain or maintain proprietary intellectual property protection; the particular prescribing preferences of physicians and patients; general political, economic and business conditions, including the effects of and efforts to mitigate pandemic diseases; safety, quality, data integrity or manufacturing issues; potential or actual data security and data privacy breaches, or disruptions of our information technology systems, and other risks and factors referred to in Novartis AG’s current Form 20-F on file with the US Securities and Exchange Commission. Novartis is providing the information in this press release as of this date and does not undertake any obligation to update any forward-looking statements contained in this press release as a result of new information, future events or otherwise.About Novartis
Novartis is an innovative medicines company. Every day, we work to reimagine medicine to improve and extend people’s lives so that patients, healthcare professionals and societies are empowered in the face of serious disease. Our medicines reach nearly 300 million people worldwide.Reimagine medicine with us: Visit us at https://www.novartis.com and connect with us on LinkedIn, Facebook, X/Twitter and Instagram.
References
- Novartis. Data on file.
- Morand E, et al. Biomarker Data From an Open-Label, Phase 1/2 Study for YTB323 (Rapcabtagene Autoleucel, a Rapidly Manufactured CD19 CAR-T Therapy) Suggest Reset of the B Cell Compartment in Severe Refractory SLE. Abstract presented at ACR Convergence 2025. Accessed September 19, 2025. https://acrabstracts.org/abstract/biomarker-data-from-an-open-label-phase-1-2-study-for-ytb323-rapcabtagene-autoleucel-a-rapidly-manufactured-cd19-car-t-therapy-suggest-reset-of-the-b-cell-compartment-in-severe-refractory-sle/
- Armstrong A, et al. Comparison of Incidence of Psoriatic Arthritis in Patients With Psoriasis Treated With Interleukin-17 Inhibitors vs Interleukin-23 Inhibitors, Interleukin-12/23 Inhibitors, and Tumor Necrosis Factor Inhibitors in Real-World Practice: A Retrospective Study. Abstract presented at ACR Convergence 2025. Accessed September 19, 2025. https://acrabstracts.org/abstract/comparison-of-incidence-of-psoriatic-arthritis-in-patients-with-psoriasis-treated-with-interleukin-17-inhibitors-vs-interleukin-23-inhibitors-interleukin-12-23-inhibitors-and-tumor-necrosis-factor-i/
- Dorner T, et al. Safety and Efficacy of ianalumab in patients with Sjogren’s disease: 52-week results from a randomized, placebo-controlled, phase 2b dose-ranging study. Arthritis and Rheumatology. 2025; 77(5):560-570
- Negrini S, et al. Sjogren’s syndrome: a systemic autoimmune disease, Clin Exp Med. 2022; 22(1): 9-25
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Selected Immune Disorders and Disability. Sjogren’s Disease/Syndrome. Accessed September 11, 2025. https://www.ncbi.nlm.nih.gov/books/NBK584486/
- ClinicalTrials.gov NCT05798117 [Last accessed: September 2025]
- ClinicalTrials.gov NCT06665256 [Last accessed: September 2025]
- ClinicalTrials.gov NCT06655896 [Last accessed: September 2025]
# # #
Continue Reading
-
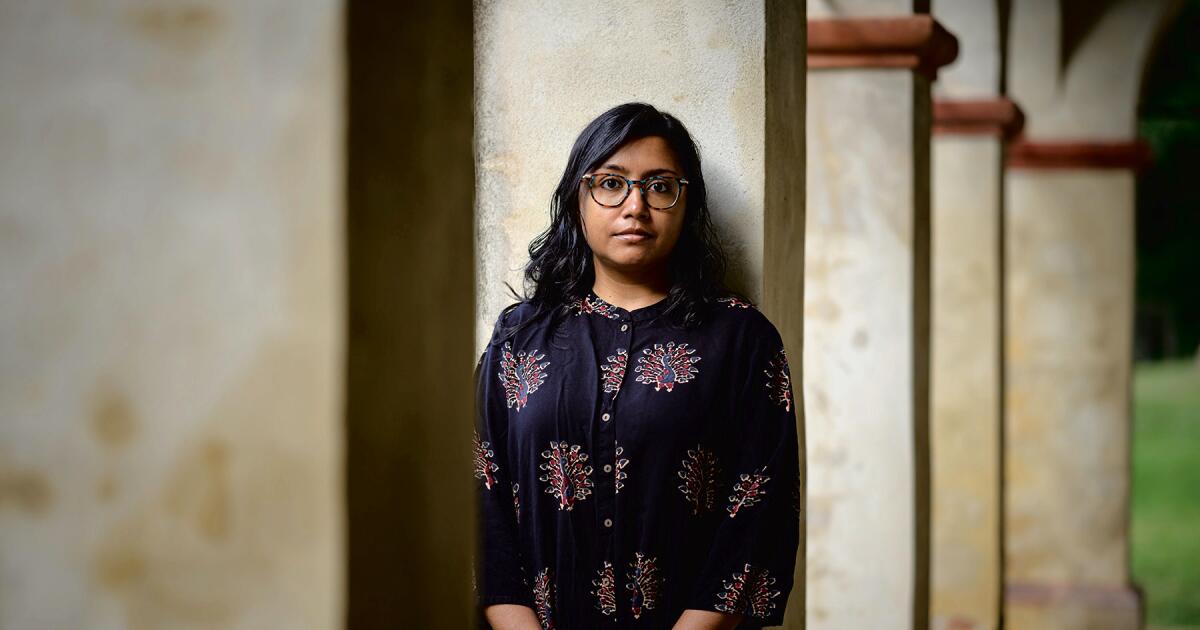
Megha Majumdar discusses her climate catastrophe book
In Megha Majumdar’s new novel “A Guardian and a Thief,” a cataclysmic climate event in the Bengali city of Kolkata has wiped out shelter and food supplies, leaving its citizens desperate and scrambling for survival. Among the families…
Continue Reading
-

Scientists uncover how some 80-year-olds have the memory of 50-year-olds
Cognitive decline is typically considered a hallmark of aging — but research suggests that not everyone is susceptible.
A study from Northwestern University looked at a group of 80-year-olds who appeared to have memory skills on par with…
Continue Reading
-
Bristol Myers Squibb Presents Encouraging Data from Phase 1 Breakfree-1 Study of CD19 NEX-T™ CAR T Cell Therapy in Three Chronic Autoimmune Diseases at ACR Convergence 2025 – Bristol Myers Squibb
- Bristol Myers Squibb Presents Encouraging Data from Phase 1 Breakfree-1 Study of CD19 NEX-T™ CAR T Cell Therapy in Three Chronic Autoimmune Diseases at ACR Convergence 2025 Bristol Myers Squibb
- Bristol Myers Squibb Presents Encouraging Data from Phase 1 Breakfree-1 Study of CD19 NEX-T Business Wire
- Bristol Myers Squibb reports positive early results from CAR T therapy trial Investing.com
Continue Reading
-

Oppo partners with Google on Find X9 series AI features in overseas markets
Oppo recently announced its Find X9 series in China and while we wait for their global launch, Oppo has announced a new partnership with Google which will debut some new AI features on the Find X9 and Find X9 Pro.
These include…
Continue Reading
-

The Droptines on Hard Work, TikTok, and Texas Country Music
The Droptines were used to playing simple dive bars and rock rooms. Their worldview changed this summer when they went on an amphitheater tour with Whiskey Myers.
By the time the Texas roots-rock band’s opening run for Whiskey Myers…
Continue Reading
-

BIMZELX® (bimekizumab-bkzx) Three-Year Rheumatology Data at ACR 2025 Demonstrated Sustained Inflammation Control in Psoriatic Arthritis and Axial Spondyloarthritis
- Sustained improvements across stringent measures of disease in patients with psoriatic arthritis (PsA): One-year improvements were sustained to three years across measures of peripheral arthritis, dactylitis, enthesitis, skin psoriasis, and nail psoriasis – indicating sustained inflammation control
- Sustained clinical response to stringent endpoints in half of patients with axial spondyloarthritis (nr-axSpA and AS): From Week 16 to three years, 50% of patients never lost ASDAS low disease activity (LDA) (<2.1) status at any assessed visit – indicating sustained inflammation control
- First real-world findings demonstrate rapid quality of life (HRQoL) improvements in patients with PsA, nr-axSpA, and AS: Improvements in outcomes in routine clinical practice were reported at 24 weeks, with benefits as early as Week 2 in some patients
ATLANTA, Oct. 25, 2025 /PRNewswire/ — UCB, a global biopharmaceutical company, today announced new three-year data from Phase 3 trials, and their open-label extensions, investigating BIMZELX® (bimekizumab-bkzx) in adults with active psoriatic arthritis (PsA), non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation, and ankylosing spondylitis (AS). BIMZELX, the first and only medicine approved to selectively inhibit both interleukin 17A (IL-17A) and interleukin 17F (IL-17F),1 continued to demonstrate sustained control of inflammation and deep efficacy in patients living with PsA, nr-axSpA, and AS.2-6
Sustained improvements across stringent measures of disease in patients with PsA2
“The diverse, multi-faceted nature of PsA can make it challenging to treat, as therapy should ideally address multiple disease domains,” said Professor Laura Coates, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Diseases, University of Oxford, United Kingdom. “These compelling data show sustained improvements over three years across key PsA disease domains. This demonstrates that bimekizumab has potential to benefit a broad range of patients, and may improve long-term inflammation control and prevent structural damage.”
In patients with PsA, one-year improvements were sustained to three years across the following GRAPPA domains:* peripheral arthritis, dactylitis, enthesitis, skin psoriasis and nail psoriasis.2 Individual domain responses were consistent between bDMARD‑naïve and TNFi-IR patients.2 Further, exposure-adjusted incidence rates per 100 patient years for uveitis and definite or probable adjudicated inflammatory bowel disease (IBD) to Week 156 were 0.2 (95% confidence interval 0.1, 0.6) and 0.3 (0.1, 0.7) in BE OPTIMAL and 0 and 0.1 (0.0, 0.6) in BE COMPLETE, respectively.2 (See Appendix for further details).
Sustained clinical response to stringent endpoints in half of patients with nr-axSpA and AS
“In clinical practice, ASDAS LDA is an important treatment target for disease control for people living with axSpA, as it is a highly stringent measure of low disease activity,” said Professor Fabian Proft, Universitätsmedizin Berlin, Germany. “It is therefore meaningful that in this study of bimekizumab, half of the patients never lost their ASDAS LDA response at any assessed visit over three years, while over three quarters of patients maintained this response over three years. This suggests long-term disease control, which is paramount in treating both nr-axSpA and AS.”
A high proportion of BIMZELX-randomized patients who achieved clinical responses at Week 16 maintained these to Week 164 across the full disease spectrum of axSpA, including nr-axSpA and AS.4 From Week 16 through to Week 164, 50% of patients never lost their ASDAS LDA (<2.1) status at any assessed visit (MI), with a further 22.4% only losing their ASDAS LDA status at one visit, and 6.1% at two visits, respectively (MI).4 Of the 152 patients (43.6%; NRI) who achieved ASDAS LDA at Week 16, 78.8% still achieved ASDAS LDA at Week 164 (MI).*4
*Proportion of patients who achieved ASDAS LDA at Week 16 and Week 164 in patients randomized to BIMZELX 160 mg every four weeks (Q4W) at baseline. 4
Real-world findings demonstrate rapid HRQoL improvements in patients with PsA, nr-axSpA, and AS5,6
Interim, post-hoc data analysis (observed case, OC) of patient-reported outcomes from the SPEAK study in routine clinical practice showed that:5,6
For BIMZELX-treated patients with PsA, improvements in PsAID-12 total score were observed to 24 weeks, with mean (SD) change from baseline (CfB) at Week 24 of −1.9 (2.0).5 SF-36 PCS scores improved to Week 24 (mean [SD] CfB: +4.6 [7.9]), as did PGADA scores (mean [SD] CfB: −17.5 [23.8]).5 At Week 2, mean (SD) CfB in PsAID-12 total score and PGADA score were -0.8 (1.6) and -7.1 (19.3), respectively.5
For BIMZELX-treated patients with nr-axSpA and AS, improvements in ASAS HI score were observed to 24 weeks, with mean (SD) CfB at Week 24 of –1.6 (3.0).6 SF-36 PCS scores improved to Week 24 (mean [SD] CfB: +5.7 [7.3]), as did PGADA scores (mean [SD] CfB: –1.0 [2.5].6 At Week 2, mean (SD) change from baseline (CfB) in ASAS HI score and PGADA score were –0.7 (2.3) and –0.8 (2.1), respectively.6
“This data presented at ACR shows that bimekizumab continues to demonstrate long-term improvement in inflammation control and deep efficacy in patients living with PsA and axSpA, and emphasizes that this effect is consistent across a spectrum of patients with these diseases,” said Donatello Crocetta, Chief Medical Officer, UCB.
UCB will present 16 abstracts on BIMZELX at ACR 2025 in Chicago, October 24-29, across nr-axSpA, AS, PsA, and psoriasis. These will complement seven other presentations from UCB across their rheumatology portfolio. This data underscores UCB’s ambition to be a leader in rheumatology, commitment to advancing clinical research and innovation, and focus on developing meaningful solutions across the spectrum of rheumatic diseases.
*Core domains of PsA according to GRAPPA (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis) recommendations.7
Study methodology
PsA abstract:2 Included patients who were randomized to subcutaneous BIMZELX 160 mg or placebo every 4 weeks (Q4W) in BE OPTIMAL (biologic DMARD [bDMARD]‑naïve patients with PsA), BE COMPLETE (patients with PsA with inadequate response or intolerance to TNF inhibitors [TNFi‑IR]), BE MOBILE 1 (nr-axSpA) and BE MOBILE 2 (AS, i.e., radiographic axSpA).2 From Week 16, all placebo-randomized patients received BIMZELX 160 mg Q4W.2 Week 52/16 BE OPTIMAL/BE COMPLETE completers were eligible for BE VITAL open-label extension; BE MOBILE 1 and 2 Week 52 completers could enter BE MOVING OLE.2
AxSpA abstract:4 BE MOBILE 1 (nr-axSpA) and 2 (AS) from Week 16, all patients received subcutaneous BIMZELX 160 mg Q4W. At Week 52, eligible patients could enroll in the OLE (BE MOVING).4
Real-world study:5,6 SPEAK is an ongoing 52-week, multi-country, observational study in Belgium, Czechia, France, Germany, Greece, Spain and the United Kingdom.5,6 This planned interim analysis reports data to April 2, 2025 (approx. 50% enrollment).5,6 Adult patients with active PsA, nr-axSpA, or AS who initiated BIMZELX in routine clinical practice could be included if receiving treatment per label (BIMZELX 160 mg Q4W).5,6
Notes to Editors
- ASAS40 responder rate: Assessment in SpondyloArthritis international Society ≥40% improvement4
- ASAS HI: Assessment of SpondyloArthritis international Society Health Index6
- ASDAS LDA: axSpA Disease Activity Score (ASDAS) low disease activity (LDA; <2.1)4,8
- Dactylitis: Inflammation of a finger or toe9
- Enthesitis: Inflammation where the tendons and ligaments insert into bones10
- GRAPPA domains: Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA)-based treatment recommendations focus on six core domains and the PsA-related conditions, uveitis and IBD. The six core domains are: peripheral arthritis, axial disease, enthesitis, dactylitis, skin psoriasis and nail psoriasis2,7
- IBD: Inflammatory Bowel Disease
- LDI: Leeds Dactylitis Index
- LEI: Leeds Enthesitis Index
- MI: Multiple imputation2
- mNAPSI: Modified nail psoriasis severity index2
- mNRI: Modified NRI2
- NRI: Non-responder imputation2
- PASI 100: 100% improvement from baseline in Psoriasis Area and Severity Index2
- PGADA: Patient Global Assessment of Disease Activity5,6
- PsAID-12: 12-item PsA Impact of Disease questionnaire5
- SF-36 PCS: Short Form 36-item Health Survey Physical Component Summary5,6
- SJC: Swollen joint count2
- TNFi-IR: Inadequate response or intolerance to tumor necrosis factor inhibitors2
- Uveitis: Inflammation of the middle layer of the eyeball called the uvea11
Appendix
Further detail from 3-year PsA data across GRAPPA domains2
For the majority of GRAPPA domains, 1-year improvements were sustained to 3 years across all studies.2 These include stringent measures of disease within the disease domains, for example, complete resolution of the following:2
Complete resolution criteria within domains2
Data extracted from abstract table for nail psoriasis, peripheral arthritis, enthesitis, dactylitis, skin psoriasis
BE OPTIMAL (bDMARD‑naïve)
BIMZELX (BKZ) Total (n=712)
BE COMPLETE (TNFi-IR)
BKZ Total (n=400)
BKZ 160 mg Q4W Total includes patients who switched from placebo to BKZ at Week 16
Year 1
Year 3
Year 1
Year 3
Peripheral arthritis2
Swollen joint count (SJC)=0 (mNRI)
61.8
(58.2−65.5)
59.5
(55.7–63.3)
58.2
(53.2−63.1)
59.1
(54.0−64.2)
% (95% CI)
Enthesitis 2
Complete resolution of enthesitis, LEI=0 (mNRI) in patients with enthesitis at baseline (LEI >0; BE OPTIMAL: n=213;
BE COMPLETE: n=142)
% (95% CI)
63.1
(56.5−69.7)
59.6
(52.7−66.5)
58.9
(50.6−67.2)
59.9
(51.4−68.4)
Dactylitis2
Complete resolution of dactylitis, LDI=0 (NRI), in patients with dactylitis at baseline (LDI >0; BE OPTIMAL: n=89;
BE COMPLETE: n=48); missing data imputed using NRI as MI was not estimable as it would
not converge
83.1
(75.4−90.9)
66.3
(56.5−76.1)
85.4
(75.4−95.4)
70.8
(58.0−83.7)
% (95% CI)
Skin psoriasis2
Complete skin clearance (PASI 100) (mNRI), in patients with
≥3% body surface area affected by psoriasis at baseline (BE OPTIMAL: n=357; BE COMPLETE: n=264)
64.7
(59.6−69.8)
61.9
(56.6−67.3)
66.2
(60.3−72.1)
67.5
61.6−73.4)
% (95% CI)
Nail psoriasis2
Complete resolution of nail psoriasis, mNAPSI=0 (mNRI), in patients with nail psoriasis at baseline (mNAPSI
>0; BE OPTIMAL: n=400;
BE COMPLETE: n=242)
68.7
(64.1−73.3)
65.6
(60.5−70.6)
67.0
(61.0−73.0)
67.1
60.9−73.4)
% (95% CI)
Axial disease2
Pooled BE MOBILE 1 and 2
nr-axSpA and ASData were pooled for all randomized patients with nr-axSpA and AS in BE MOBILE 1 and 2
Any BKZ 160 mg Q4W
Includes patients who switched from placebo to BKZ at Week 16
n=586
Year 1
Year 3
ASAS40 responder rate (MI),
% (95% CI)
62.4 (58.4, 66.4)
60.2
(56.0, 64.5)
Table adapted from data extracted from abstract table for nail psoriasis, peripheral arthritis, enthesitis, dactylitis, skin psoriasis, axial disease.
About Psoriatic Arthritis
Psoriatic arthritis is a serious, highly heterogeneous, chronic, systemic inflammatory condition affecting both the joints and skin with a prevalence of 0.02 percent to 0.25 percent of the population.12 Psoriatic arthritis affects approximately 30 percent of people living with psoriasis.13 It manifests as joint pain and stiffness, skin plaques, swollen toes and fingers (dactylitis) and inflammation of the sites where tendons or ligaments insert into the bone (enthesitis).14 The burden on those living with PsA extends beyond physical discomfort to reduced quality of life, with comorbidities including hypertension, cardiovascular disease, anxiety, and depression.15 In PsA, uncontrolled active disease can lead to long-term, irreversible structural damage.16
About BE OPTIMAL and BE COMPLETE
BE OPTIMAL and BE COMPLETE were two Phase 3 studies evaluating the efficacy and safety of BIMZELX in the treatment of psoriatic arthritis.17,18 The primary endpoint in both studies was the proportion of patients reaching 50% or greater improvement in American College of Rheumatology criteria (ACR50) at Week 16.17,18 BE OPTIMAL (bDMARD-naïve) and BE COMPLETE (TNFi-IR) assessed subcutaneous BIMZELX 160 mg every four weeks (Q4W) in patients with PsA; both studies were placebo-controlled to Week 16, after which placebo patients switched to BIMZELX.17,18
BE OPTIMAL Week 52 and BE COMPLETE Week 16 completers were eligible for BE VITAL open-label extension.17,18
About Axial Spondyloarthritis
Axial spondyloarthritis (axSpA), which includes both non-radiographic axSpA (nr-axSpA) and ankylosing spondylitis (AS), is a chronic, immune-mediated, inflammatory disease.19 nr-axSpA is defined clinically by the absence of definitive x-ray evidence of structural damage to the sacroiliac joints.19 axSpA is a painful condition that primarily affects the spine and the joints linking the pelvis and lower spine (sacroiliac joints).19 The leading symptom of axSpA in a majority of patients is inflammatory back pain that improves with exercise, but not with rest.19 Other common clinical features frequently include anterior uveitis, enthesitis, peripheral arthritis, psoriasis, inflammatory bowel disease, and dactylitis.19 The overall prevalence of axSpA is 0.3 percent to 1.4 percent of adults.20,21 Approximately half of all patients with axSpA are patients with nr-axSpA.19 axSpA onset usually occurs before the age of 45.19 Approximately 10 to 40 percent of patients with nr-axSpA progress to ankylosing spondylitis over 2 to 10 years.19
About BE MOBILE 1 and BE MOBILE 2
BE MOBILE 1 and BE MOBILE 2 were two Phase 3 studies evaluating the efficacy and safety of BIMZELX in the treatment of nr-axSpA and AS, respectively.22 The primary endpoint in both studies was the Assessment of SpondyloArthritis international Society 40 percent (ASAS40) response at Week 16.22 BE MOBILE 1 and BE MOBILE 2 comprised a 16-week double-blind treatment period followed by a 36-week maintenance period.22 In BE MOBILE 1 and BE MOBILE 2, patients were randomized to BIMZELX (160 mg Q4W; N=128 for BE MOBILE 1 and N=221 for BE MOBILE 2) or to placebo (N=126 for BE MOBILE 1 and N=111 for BE MOBILE 2). Patients initially randomized to placebo were switched to BIMZELX (160 mg Q4W) at Week 16.22 BE MOBILE 1 and BE MOBILE 2 Week 52 completers were eligible for BE MOVING open-label extension.22
About BIMZELX® (bimekizumab-bkzx)
BIMZELX is a humanized monoclonal IgG1 antibody that is designed to selectively inhibit both interleukin 17A (IL-17A) and interleukin 17F (IL-17F), two key cytokines driving inflammatory processes.1 IL-17A and IL-17F are key contributors of chronic inflammation and damage across multiple tissues, with IL-17F increasing over time.1,23-25 IL-17F is over-expressed in skin and highly elevated in the serum of patients with psoriasis (PSO), psoriatic arthritis (PsA), non-radiographic axial spondyloarthritis (nr-axSpA), ankylosing spondylitis (AS), and hidradenitis suppurativa (HS).1,23-26The approved indications for BIMZELX in the U.S. are:1
- Plaque psoriasis: BIMZELX is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy
- Psoriatic arthritis: BIMZELX is indicated for the treatment of adult patients with active psoriatic arthritis
- Non-radiographic axial spondyloarthritis: BIMZELX is indicated for the treatment of adult patients with active non-radiographic axial spondyloarthritis with objective signs of inflammation
- Ankylosing spondylitis: BIMZELX is indicated for the treatment of adult patients with active ankylosing spondylitis
- Hidradenitis suppurativa: BIMZELX is indicated for the treatment of adults with moderate- to-severe hidradenitis suppurativa
BIMZELX U.S. IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
Suicidal Ideation and Behavior
BIMZELX (bimekizumab-bkzx) may increase the risk of suicidal ideation and behavior (SI/B). A causal association between treatment with BIMZELX and increased risk of SI/B has not been definitively established. Prescribers should weigh the potential risks and benefits before using BIMZELX in patients with a history of severe depression or SI/B. Advise monitoring for the emergence or worsening of depression, suicidal ideation, or other mood changes. If such changes occur, instruct to promptly seek medical attention, refer to a mental health professional as appropriate, and re- evaluate the risks and benefits of continuing treatment.Infections
BIMZELX may increase the risk of infections, including serious infections. Do not initiate treatment with BIMZELX in patients with any clinically important active infection until the infection resolves or is adequately treated. In patients with a chronic infection or a history of recurrent infection, consider the risks and benefits prior to prescribing BIMZELX. Instruct patients to seek medical advice if signs or symptoms suggestive of clinically important infection occur. If a patient develops such an infection or is not responding to standard therapy, monitor the patient closely and do not administer BIMZELX until the infection resolves.Tuberculosis
Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with BIMZELX. Avoid the use of BIMZELX in patients with active TB infection. Initiate treatment of latent TB prior to administering BIMZELX. Consider anti-TB therapy prior to initiation of BIMZELX in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Closely monitor patients for signs and symptoms of active TB during and after treatment.Liver Biochemical Abnormalities
Elevated serum transaminases were reported in clinical trials with BIMZELX. Test liver enzymes, alkaline phosphatase, and bilirubin at baseline, periodically during treatment with BIMZELX, and according to routine patient management. If treatment-related increases in liver enzymes occur and drug-induced liver injury is suspected, interrupt BIMZELX until a diagnosis of liver injury is excluded. Permanently discontinue use of BIMZELX in patients with causally associated combined elevations of transaminases and bilirubin. Avoid use of BIMZELX in patients with acute liver disease or cirrhosis.Inflammatory Bowel Disease
Cases of inflammatory bowel disease (IBD) have been reported in patients treated with IL-17 inhibitors, including BIMZELX. Avoid use of BIMZELX in patients with active IBD. During BIMZELX treatment, monitor patients for signs and symptoms of IBD and discontinue treatment if new onset or worsening of signs and symptoms occurs.Immunizations
Prior to initiating therapy with BIMZELX, complete all age-appropriate vaccinations according to current immunization guidelines. Avoid the use of live vaccines in patients treated with BIMZELX.Most Common Adverse Reactions
Most common (≥ 1%) adverse reactions in plaque psoriasis and hidradenitis suppurativa include upper respiratory tract infections, oral candidiasis, headache, injection site reactions, tinea infections, gastroenteritis, herpes simplex infections, acne, folliculitis, other candida infections, and fatigue.Most common (≥ 2%) adverse reactions in psoriatic arthritis include upper respiratory tract infections, oral candidiasis, headache, diarrhea, and urinary tract infections.
Most common (≥ 2%) adverse reactions in non-radiographic axial spondyloarthritis include upper respiratory tract infections, oral candidiasis, headache, diarrhea, cough, fatigue, musculoskeletal pain, myalgia, tonsillitis, transaminase increase, and urinary tract infections.
Most common (≥ 2%) adverse reactions in ankylosing spondylitis include upper respiratory tract infections, oral candidiasis, headache, diarrhea, injection site pain, rash, and vulvovaginal mycotic infection.
Please see Important Safety Information below and full U.S. Prescribing Information at www.UCB- USA.com/Innovation/Products/BIMZELX.
For further information, contact UCB:
Investor Relations
Antje Witte
T +32.2.559.94.14
email [email protected]Brand Communications
Nicole Herga
T +1.773.960.5349
email: [email protected]About UCB
UCB, Brussels, Belgium (www.ucb.com) is a global biopharmaceutical company focused on the discovery and development of innovative medicines and solutions to transform the lives of people living with severe diseases of the immune system or of the central nervous system. With approximately 9,000 people in approximately 40 countries, the company generated revenue of €6.1 billion in 2024. UCB is listed on Euronext Brussels (symbol: UCB). Follow us on Twitter:@UCBUSA.Forward looking statements
This document contains forward-looking statements, including, without limitation, statements containing the words “potential”, “believes”, “anticipates”, “expects”, “intends”, “plans”, “seeks”, “estimates”, “may”, “will”, “continue” and similar expressions. These forward-looking statements are based on current plans, estimates and beliefs of management. All statements, other than statements of historical facts, are statements that could be deemed forward-looking statements, including estimates of revenues, operating margins, capital expenditures, cash, other financial information, expected legal, arbitration, political, regulatory or clinical results or practices and other such estimates and results. By their nature, such forward-looking statements are not guaranteeing future performance and are subject to known and unknown risks, uncertainties, and assumptions which might cause the actual results, financial condition, performance or achievements of UCB, or industry results, to be materially different from any future results, performance, or achievements expressed or implied by such forward-looking statements contained in this document.Important factors that could result in such differences include but are not limited to: global spread and impacts of wars, pandemics and terrorism, the general geopolitical environment, climate change, changes in general economic, business and competitive conditions, the inability to obtain necessary regulatory approvals or to obtain them on acceptable terms or within expected timing, costs associated with research and development, changes in the prospects for products in the pipeline or under development by UCB, effects of future judicial decisions or governmental investigations, safety, quality, data integrity or manufacturing issues, supply chain disruption and business continuity risks; potential or actual data security and data privacy breaches, or disruptions of UCB’s information technology systems, product liability claims, challenges to patent protection for products or product candidates, competition from other products including biosimilars or disruptive technologies/business models, changes in laws or regulations, exchange rate fluctuations, changes or uncertainties in laws and/or rules pertaining to tax and duties or the administration of such laws and/or rules, and hiring, retention and compliance of employees. There is no guarantee that new product candidates will be discovered or identified in the pipeline, or that new indications for existing products will be developed and approved. Movement from concept to commercial product is uncertain; preclinical results do not guarantee safety and efficacy of product candidates in humans. So far, the complexity of the human body cannot be reproduced in computer models, cell culture systems or animal models. The length of the timing to complete clinical trials and to get regulatory approval for product marketing has varied in the past and UCB expects similar unpredictability going forward. Products or potential products which are the subject of partnerships, joint ventures or licensing collaborations may be subject to disputes between the partners or may prove to be not as safe, effective or commercially successful as UCB may have believed at the start of such partnership. UCB’s efforts to acquire other products or companies and to integrate the operations of such acquired companies may not be as successful as UCB may have believed at the moment of acquisition. Also, UCB or others could discover safety, side effects or manufacturing problems with its products and/or devices after they are marketed. The discovery of significant problems with a product similar to one of UCB’s products that implicate an entire class of products may have a material adverse effect on sales of the entire class of affected products. Moreover, sales may be impacted by international and domestic trends toward managed care and health care cost containment, including pricing pressure, political and public scrutiny, customer and prescriber patterns or practices, and the reimbursement policies imposed by third-party payers as well as legislation affecting biopharmaceutical pricing and reimbursement activities and outcomes. Finally, a breakdown, cyberattack or information security breach could compromise the confidentiality, integrity and availability of UCB’s data and systems.
Given these uncertainties, the public is cautioned not to place any undue reliance on such forward-looking statements. These forward- looking statements are made only as of the date of this document, and do not reflect any potential impacts from the evolving event or risk as mentioned above as well as any other adversity, unless indicated otherwise. The company continues to follow the development diligently to assess the financial significance of these events, as the case may be, to UCB.
UCB expressly disclaims any obligation to update any forward-looking statements in this document, either to confirm the actual results or to report or reflect any change in its forward-looking statements with regard thereto or any change in events, conditions or circumstances on which any such statement is based, unless such statement is required pursuant to applicable laws and regulations.
References
- BIMZELX® (bimekizumab-bkzx) U.S. Prescribing Information. https://www.ucb-usa.com/Innovation/Products/BIMZELX.
- Merola J. Sustained Efficacy up to 3 Years with Bimekizumab Treatment Across GRAPPA Core Domains in Patients with Psoriatic Arthritis: Long-Term Results from Two Phase 3 Trials [abstract]. ACR 2025. #2129566.
- Rudwaleit M. Long-Term Uveitis Rates with Bimekizumab Treatment Across Pooled Phase 2b and Phase 3 Studies in Patients with Axial Spondyloarthritis or Psoriatic Arthritis: 3-Year Update [abstract]. ACR 2025. #2129301.
- Proft F. Bimekizumab Treatment Resulted in Patients with Axial Spondyloarthritis Maintaining Their Clinical Responses Over 3 Years: Results from Two Phase 3 Studies and Their Open-Label Extension [abstract].ACR 2025. #2129239.
- Baraliakos X. An Interim Analysis of Health-Related Quality of Life Outcomes from an International Multicentre Observational Study in Patients with Psoriatic Arthritis Initiating Bimekizumab in Real-World Clinical Practice [abstract]. ACR 2025. #2127676.
- Baraliakos X. An Interim Analysis of Health-Related Quality of Life Outcomes from an International Multicentre Observational Study in Patients with Axial Spondyloarthritis Initiating Bimekizumab in Real-World Clinical Practice. [abstract]. ACR 2025. #2127714.
- Coates LC, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): Updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18(8):465–79.
- Garcia-Magallón B et al. Is the new ASDAS nomenclature in agreement with therapeutic decision making in patients with axial spondyloarthritis? Semin Arthritis and Rheum. 2020;50(5):854-57.
- Hamard A, Burns R, Miquel A, et al. Dactylitis: A pictorial review of key symptoms. Diagn Interv Imaging. 2020;101(4):193-207.
- Sudoł-Szopińska I, Kwiatkowska B, Prochorec-Sobieszek M, et al. Enthesopathies and enthesitis. Part 1. Etiopathogenesis. J Ultrason. 2015;15(60):72-84.
- Maghsoudlou P, Epps SJ, Guly CM, et al. Uveitis in adults: a review. JAMA. 2025;334(5):419-434.
- Ogdie A, Weiss P. The Epidemiology of Psoriatic Arthritis. Rheum Dis Clin North Am. 2015;41(4):545–68.
- Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69(5):729-735.
- Mease PJ, Armstrong A. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74(4):423–41.
- Lee S, Mendelsohn A, Sarnes E. The burden of psoriatic arthritis: A literature review from a global health systems perspective. P T. 2010;35(12):680–89.
- Kwok T, Sutton M, Cook R, et al. Musculoskeletal Surgery in Psoriatic Arthritis: Prevalence and Risk Factors. J Rheumatol. 2023;50(4):497–503.
- Ritchlin C, Coates L, McInnes I, et al. Bimekizumab treatment in biologic DMARD-naïve patients with active psoriatic arthritis: 52-week efficacy and safety results from the phase III, randomised, placebo-controlled, active reference BE OPTIMAL study. Ann Rheum Dis. 2023;82(11);1404–14.
- Coates L, Landewé R, McInnes I, et al. Bimekizumab treatment in patients with active psoriatic arthritis and prior inadequate response to tumour necrosis factor inhibitors: 52-week safety and efficacy from the phase III BE COMPLETE study and its open-label extension BE VITAL. RMD Open. 2024;10(1):e003855.
- Deodhar A. Understanding Axial Spondyloarthritis: A Primer for Managed Care. Am J Manag Care. 2019;25(1):S319–30.
- Reveille J, Witter J, Weisman M. Prevalence of axial spondyloarthritis in the United States: estimates from a cross- sectional survey. Arthritis Care Res (Hoboken). 2012;64(6):905–10.
- Hamilton L, Macgregor A, Toms A, et al. The prevalence of axial spondyloarthritis in the UK: a cross-sectional cohort study. BMC Musculoskelet Disord. 2015;16:392.
- Baraliakos X, Deodhar A, van der Heijde D, et al. Bimekizumab treatment in patients with active axial spondyloarthritis: 52-week efficacy and safety from the randomised parallel phase 3 BE MOBILE 1 and BE MOBILE 2 studies. Ann Rheum Dis. 2024;83(2):199–213.
- Glatt S, Baeten D, Baker T et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523–32.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate-to-severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397(10273):475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate-to-severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397(10273):487-498.
- Rumberger BE, Boarder EL, Owens SL, et al. Transcriptomic analysis of hidradenitis suppurativa skin suggests roles for multiple inflammatory pathways in disease pathogenesis. Inflamm Res. 2020;69(10):967-973.
US-BK-2501221
Date of preparation: October 2025
BIMZELX® is a registered trademark of the UCB Group of Companies.
©2025 UCB, Inc., Smyrna, GA 30080. All rights reservedSOURCE UCB
Continue Reading

