- Thermo Fisher to buy clinical services provider Clario for up to $9.4 billion Reuters
- Thermo Fisher nears $10bn takeover of drug trial software maker Clario Financial Times
- Thermo Fisher Scientific to acquire Clario Holdings, enabling pharma and biotech customers to accelerate innovation with deeper clinical insights MarketScreener
- Thermo Fisher Scientific to Acquire Clario Holdings, Inc., Enabling Pharma and Biotech Customers to Accelerate Innovation with Deeper Clinical Insights Business Wire
- M&A News: Thermo Fisher (TMO) Nears $10B Clario Takeover to Strengthen Clinical Trial Tech TipRanks
Blog
-
Thermo Fisher to buy clinical services provider Clario for up to $9.4 billion – Reuters
-

Donald Trump says he has ‘pretty much’ reached a trade deal with South Korea
Unlock the White House Watch newsletter for free
Your guide to what Trump’s second term means for Washington, business and the world
Donald Trump has said that he has “pretty much finalised” a deal with South Korea, as the two countries seek…
Continue Reading
-

Cuba Gooding Jr. Joins London-Shot Thriller ‘Angels In Darkness’
EXCLUSIVE: Cuba Gooding Jr. has joined the cast of Ali Zamani’s neo-noir fantasy thriller, Angels In Darkness, which is now filming across multiple locations in London.
The film reunites Gooding Jr. and Zamani following their…
Continue Reading
-

3D DNA Looping Boosts Rice Yields, Cuts Fertilizer Use
A team of Chinese scientists has uncovered a hidden 3D structure in rice DNA that allows the crop to grow more grain while using less nitrogen fertilizer. The finding, published in Nature Genetics by researchers from the Chinese Academy…
Continue Reading
-

Diabetes Linked to Higher Risk of Late-Onset Schizophrenia
A NATIONWIDE cohort study has revealed that adults diagnosed with type 2 diabetes (T2D) in midlife and old age face a significantly increased risk of developing very late-onset schizophrenia. The research, conducted in Israel, followed nearly…
Continue Reading
-
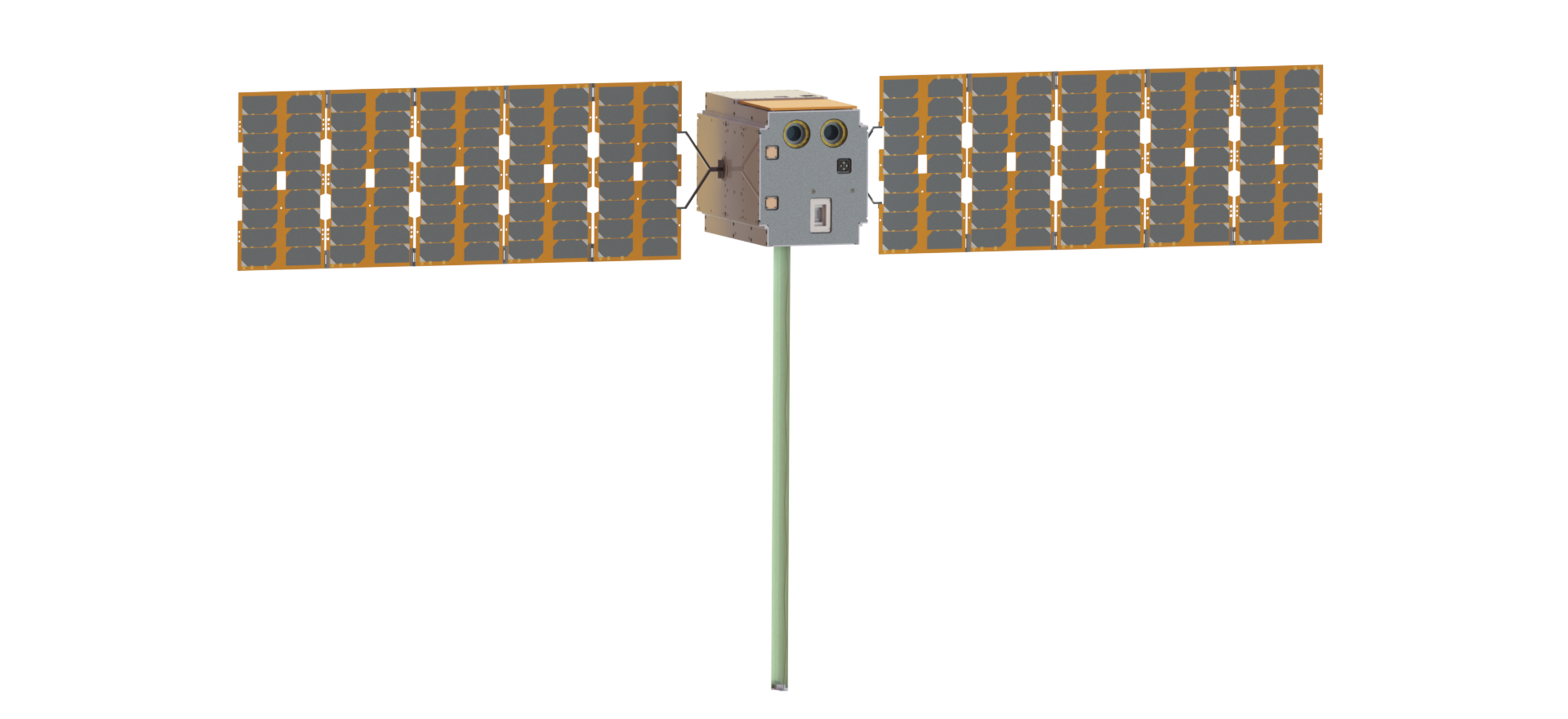
ESA’s first stand-alone deep-space CubeSat Henon takes shape
Enabling & Support 29/10/2025
20 views
0 likesThe European Space Agency’s upcoming Henon mission will be the first ever CubeSat to independently venture into deep space, communicate with Earth…
Continue Reading
-

Suspected Nation-State Threat Actor Uses New Airstalk Malware in a Supply Chain Attack
Executive Summary
We have discovered a new Windows-based malware family we’ve named Airstalk, which is available in both PowerShell and .NET variants. We assess with medium confidence that a possible nation-state threat actor…
Continue Reading
-

Back on track: how lockdown led to a new operatic version of The Railway Children | Opera
I have history with Glyndebourne. Back in 1984 when I was a naive 24-year-old, I was a music copyist to make ends meet. My great friend and teacher Oliver Knussen got me work on his opera Where the Wild Things Are ahead of its premiere by…
Continue Reading
-

NLS Pharmaceutics Ltd. Announces 1-for-10 Reverse Share Split and Name Change in Connection with Proposed Merger with Kadimastem
ZURICH, Oct. 29, 2025 /PRNewswire/ — NLS Pharmaceutics Ltd. (NASDAQ: NLSP) (NASDAQ: NLSPW) (“NLS” or the “Company”), a Swiss clinical-stage biopharmaceutical company focused on the discovery and development of innovative therapies for patients with rare and complex central nervous system disorders, today announced that it intends to effect a reverse share split of the Company’s issued and outstanding common shares, par value CHF 0.03 per share (the “Common Shares”), at a ratio of 1-for-10 (the “Reverse Split”). The Company’s Common Shares are expected to begin trading on the Nasdaq Capital Market on a post-split basis at the market open on October 31, 2025, under the new trading symbol “NCEL”, and new name, NewcelX Ltd., following the anticipated closing of the merger (the “Merger”) with Kadimastem Ltd. (TASE: KDST) (“Kadimastem”).
The new CUSIP number for the Common Shares following the Merger and the Reverse Split will be H5835A109. A notice of the Reverse Split and other capital measures approved by the Company in connection with the Merger and the Reverse Split, will be filed with the commercial registry of the Canton of Zurich, Switzerland, on October 30, 2025. As a result, the Reverse Split will become effective in Switzerland on October 30, 2025, and will be reflected on Nasdaq at market open on October 31, 2025. The reverse split will be published in the Swiss Official Gazette of Commerce (SOGC) on or around October 31, 2025.
The Reverse Split also included a reverse split of the Company’s preferred shares, par value CHF 0.03 and preferred participation certificates, par value CHF 0.03, at a ratio of 1-for-10. The Reverse Split and other capital measures in connection with the Merger and the Reverse Split were approved at the Company’s extraordinary shareholders’ meeting held on September 29, 2025.
After giving effect to the Reverse Split and the Merger, the Company’s registered capital shall consist of: (A) (i) a total share capital of CHF 282,908.80, divided into 5,533,183 common shares, par value CHF 0.05 each, and 124,993 preferred shares, par value CHF 0.05 each, and (ii) a total participation capital of CHF 3,032.40, divided into 60,648 preferred participation certificates, par value CHF 0.05 each; (B) a capital band with an upper limit of CHF 428,911.80 and a lower limit of CHF 142,970.60, which may be effected by issuing up to 2,859,412 fully paid registered common shares, par value CHF 0.05 each; and (C) conditional share capital consisted of (i) a maximum of 450,000 fully paid in registered common shares, par value CHF 0.05 each which amounts to CHF 22,500, and (ii) a maximum amount of CHF 120,470.60 through the issuance of not more than 2,409,412 registered common shares, par value CHF 0.05 each. The foregoing description of the Amended and Restated Articles of Association is qualified in its entirety by reference to the Amended and Restated Articles of Association filed as Exhibit 99.1 hereto, which is incorporated herein by reference.
No fractional shares will be issued as a result of the Reverse Split and cash in lieu will be provided for any fractional shares resulting from the Reverse Split on a per shareholder basis. The Reverse Split will not impact any shareholder’s percentage ownership of NLS or voting power, except for minimal effects resulting from the treatment of fractional shares. All options and warrants of the Company outstanding prior to the split will be appropriately adjusted.
Following the closing of the Reverse Split and the Merger, the Company is expected to have (i) 4,558,378 total outstanding common shares, par value CHF 0.05, (ii) 1,060,574 total outstanding common shares, par value CHF 0.05, issuable upon the exercise of pre-funded warrants issued as Merger consideration, (iii) 13,778 total outstanding preferred shares, par value CHF 0.05, and (iv) 58,320 total outstanding preferred participation certificates, par value CHF 0.05.
VStock Transfer, LLC, will act as the exchange agent for the Reverse Split. Please contact VStock Transfer, LLC for further information at (212) 828-8436.
About NLS Pharmaceutics Ltd.
NLS Pharmaceutics Ltd. (Nasdaq: NLSP) is a Swiss-based biopharmaceutical company focused on the development of innovative therapies for central nervous system disorders and related indications. For more information, visit www.nlspharma.com.
About Kadimastem Ltd.
Kadimastem Ltd. (TASE: KDST) is a clinical-stage cell therapy company developing allogeneic, “off-the-shelf” cell products for neurodegenerative diseases and diabetes. For more information, visit www.kadimastem.com.
Social Media: LinkedIn, X, Facebook
Safe Harbor Statement
This press release contains expressed or implied forward-looking statements pursuant to U.S. Federal securities laws. For example, the Company is using forward-looking statements when discussing the implementation, and proposed timing, of the Reverse Split, as well as the expected closing of the Merger and the potential benefits of the Merger to NLS and Kadimastem and their respective shareholders. These forward-looking statements and their implications are based on the current expectations of the management of NLS only and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. The following factors, among others, could cause actual results to differ materially from those described in the forward-looking statements: risks related to the companies’ ability to complete the Merger on the proposed terms and schedule, including risks and uncertainties related to the satisfaction of the closing conditions related to the merger agreement; and unexpected costs, charges or expenses resulting from the transaction and potential adverse reactions or changes to business relationships resulting from the announcement or completion of the proposed Merger. Except as otherwise required by law, NLS undertakes no obligation to publicly release any revisions to these forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. More detailed information about the risks and uncertainties affecting NLS is contained under the heading “Risk Factors” in NLS’ annual report on Form 20-F for the year ended December 31, 2024, filed with the Securities and Exchange Commission, or SEC, which is available on the SEC’s website, www.sec.gov, and in subsequent filings made by NLS with the SEC.
For additional information:
Investor & Media Contacts
NLS Contacts:
[email protected]
www.nlspharma.comKadimastem Contacts:
Sarah Bazak, Investors relations
[email protected]
www.kadimastem.comLogo: https://mma.prnewswire.com/media/2637716/NLS_Pharmaceutics_Logo.jpg
SOURCE NLS Pharmaceutics Ltd.; Kadimastem Ltd.
Continue Reading
-

Theravance Biopharma to Present Data on Ampreloxetine at the 36th International Symposium on The Autonomic Nervous System
- Ampreloxetine clinical development program to be featured in four presentations at the upcoming International Symposium on The Autonomic Nervous System
- Topline results from the ongoing Phase 3 CYPRESS study of ampreloxetine anticipated in Q1 2026
DUBLIN, Oct. 29, 2025 /PRNewswire/ — Theravance Biopharma, Inc. (NASDAQ: TBPH) today announced that it will be participating at the 36th International Symposium on the Autonomic Nervous System, organized by the American Autonomic Society (AAS), taking place November 5-8, 2025, in Clearwater Beach, FL. The Company will have one platform presentation and three poster presentations on its clinical development program for ampreloxetine, a potential first-in-class norepinephrine reuptake inhibitor in development for the treatment of symptomatic neurogenic orthostatic hypotension (nOH) symptoms due to multiple system atrophy (MSA).
“We are excited to have a strong presence at the upcoming International Symposium on the Autonomic Nervous System. Our platform presentation will highlight results from the previous REDWOOD trial, where we observed a durable symptomatic nOH benefit with improvement in activities of daily living in the pre-specified subgroup analysis in patients with MSA treated with ampreloxetine1,” commented Lucy Norcliffe-Kaufmann, Ph.D., Theravance Biopharma’s Executive Director of Clinical Science. “Additionally, we are pleased to share the rigorous methodologies we developed based on our previous trial experience to support enrollment and patient retention. By applying these insights, we were well positioned to successfully address the executional challenges associated with clinical studies in rare and severe neurodegenerative diseases.”
Presentations information and key findings:
Platform presentation: Precision therapy with ampreloxetine for neurogenic orthostatic hypotension in multiple system atrophy
Presenter: Lucy Norcliffe-Kaufmann, Ph.D.; Theravance Biopharma’s Executive Director of Clinical Science and Associate Professor, New York University Langone Health Dysautonomia Center
Session date and time: Saturday, November 8, 2025, at 8:30 am ETThe following three posters will be presented at Poster Session II, taking place on Thursday, November 6, 2025, at 7:00-8:30 pm ET:
Poster title: The impact of ampreloxetine on supine hypertension: an ambulatory blood pressure monitoring study
Presenter: Lucy Norcliffe-Kaufmann, Ph.D.; Theravance Biopharma’s Executive Director of Clinical Science and Associate Professor, New York University Langone Health Dysautonomia Center
Poster number: 9Poster title: Retention strategies in rare disease clinical trials: a case study of symptomatic treatment for multiple system atrophy
Presenter: Molly Szpyhulsky, MBA, BSN, RN; Theravance Biopharma U.S.
Poster number: 15Poster title: Enrollment strategies in a phase III trial for MSA-related nOH: insights from global investigators
Presenter: Molly Szpyhulsky, MBA, BSN, RN; Theravance Biopharma U.S.
Poster number: 10About Ampreloxetine
Ampreloxetine, an investigational, once-daily, selective norepinephrine reuptake inhibitor in development for the treatment of symptomatic neurogenic orthostatic hypotension (nOH) in patients with multiple system atrophy (MSA). The unique benefits of ampreloxetine treatment reported in MSA patients from Study 0170 included an increase in norepinephrine levels, a favorable impact on blood pressure, clinically meaningful and durable symptom improvement, and no signal for worsening of supine hypertension. In the US, the Company has been granted an Orphan Drug Designation for ampreloxetine for the treatment of symptomatic nOH in patients with MSA and, if results from the ongoing Phase 3 CYPRESS study are supportive, plans to file an NDA for full approval in this indication.About the Phase 3 CYPRESS (Study 0197) Study
The CYPRESS Study (NCT05696717) is a registrational Phase 3, multi-center, randomized withdrawal study to evaluate the efficacy and durability of ampreloxetine in participants with MSA and symptomatic nOH after 20 weeks of treatment; the primary endpoint of the study is change in the Orthostatic Hypotension Symptom Assessment (OHSA) composite score. The Study includes four periods: screening, open label (12-week period, participants will receive a single daily 10 mg dose of ampreloxetine), randomized withdrawal (eight-week period, double-blind, placebo-controlled, participants will receive a single daily 10 mg dose of placebo or ampreloxetine), and a long-term treatment extension. Secondary outcome measures include change from baseline in Orthostatic Hypotension Daily Activity Scale (OHDAS) item 1 (activities that require standing for a short time) and item 3 (activities that require walking for a short time).About the Phase 3 SEQUOIA (Study 0169) and REDWOOD (Study 0170) Studies
Study 0169 (NCT03750552) was a Phase 3, 4-week, multi-center, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of ampreloxetine compared to placebo in patients with symptomatic nOH (n=195). Patients from Study 0169 were eligible to enter into Study 0170 (NCT03829657), a Phase 3, multi-center, 22-week study comprising a 16-week open-label period and a 6-week double-blind, placebo-controlled, randomized withdrawal period to evaluate the sustained benefit in efficacy and safety of ampreloxetine in patients with symptomatic nOH. The primary endpoint for Study 0170 of treatment failure at week 6 was defined as a worsening of both Orthostatic Hypotension Symptom Assessment Scale (OHSA) question #1 and Patient Global Impression of Severity (PGI-S) scores by 1.0 point. After Study 0169 did not meet its primary endpoint, the Company took actions to close out the ongoing clinical program including Study 0170. The study was more than 80% enrolled (n=128/154 planned) despite stopping early. The primary endpoint was not statistically significant for the overall population of patients, which included patients with Parkinson’s disease, pure autonomic failure and MSA (odds ratio=0.6; p-value=0.196). The pre-specified subgroup analysis by disease type suggests the benefit seen in patients receiving ampreloxetine was largely driven by MSA patients (n=40). An odds ratio of 0.28 (95% CI: 0.05, 1.22) was observed in MSA patients indicating a 72% reduction in the odds of treatment failure with ampreloxetine compared to placebo. The benefit to MSA patients was observed in multiple endpoints including OHSA composite, Orthostatic Hypotension Daily Activities Scale (OHDAS) composite, Orthostatic Hypotension Questionnaire (OHQ) composite and OHSA #1.1About Multiple System Atrophy (MSA) and Symptomatic Neurogenic Orthostatic Hypotension (nOH)
MSA is a progressive brain disorder that affects movement and balance and disrupts the function of the autonomic nervous system. The autonomic nervous system controls body functions that are mostly involuntary. One of the most frequent autonomic symptoms associated with MSA is a sudden drop in blood pressure upon standing (nOH).2 There are approximately 50,000 MSA patients in the US3 and 70-90% of MSA patients experience nOH symptoms.4 Despite available therapies, many MSA patients remain symptomatic with nOH.5Neurogenic orthostatic hypotension (nOH) is a rare disorder defined as a fall in systolic blood pressure of ⩾20 mm Hg or diastolic blood pressure of ⩾10 mm Hg, within 3 minutes of standing. Severely affected patients are unable to stand for more than a few seconds because of their decrease in blood pressure, leading to cerebral hypoperfusion and syncope. A debilitating disorder, nOH results in a range of symptoms including dizziness, lightheadedness, fainting, fatigue, blurry vision, weakness, trouble concentrating, and head and neck pain.
About Theravance Biopharma
Theravance Biopharma, Inc.’s focus is to deliver Medicines that Make a Difference® in people’s lives. In pursuit of its purpose, Theravance Biopharma leverages decades of expertise, which has led to the development of FDA-approved YUPELRI® (revefenacin) inhalation solution indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD). Ampreloxetine, its late-stage investigational once-daily norepinephrine reuptake inhibitor in development for symptomatic neurogenic orthostatic hypotension (nOH) in patients with Multiple System Atrophy (MSA), has the potential to be a first in class therapy effective in treating a constellation of cardinal symptoms in MSA patients. The Company is committed to creating/driving shareholder value.For more information, please visit www.theravance.com.
THERAVANCE BIOPHARMA®, THERAVANCE® and the Cross/Star logo are registered trademarks of the Theravance Biopharma group of companies (in the U.S. and certain other countries).YUPELRI® is a registered trademark of Viatris Specialty LLC. Trademarks, trade names or service marks of other companies appearing on this press release are the property of their respective owners.
Forward-Looking Statements
This press release will contain certain “forward-looking” statements as that term is defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, statements relating to goals, plans, objectives, expectations and future events. Theravance Biopharma, Inc. (the “Company”) intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934, as amended, and the Private Securities Litigation Reform Act of 1995. Examples of such statements include statements relating to: the Company’s expectations regarding its future profitability, expenses and uses of cash, the Company’s goals, designs, strategies, plans and objectives, future growth of YUPELRI sales, future royalty payments, the ability to provide value to shareholders, the Company’s regulatory strategies and timing of clinical studies, possible safety, efficacy or differentiation of our investigational therapy, the status of patent infringement litigation initiated by the Company and its partner against certain generic companies in federal district courts; contingent payments due to the Company from the sale of the Company’s TRELEGY ELLIPTA royalty interests to Royalty Pharma, and expectations around the use of OHSA scores as endpoints for clinical trials. These statements are based on the current estimates and assumptions of the management of Theravance Biopharma as of the date of this press release and the conference call and are subject to risks, uncertainties, changes in circumstances, assumptions and other factors that may cause the actual results of Theravance Biopharma to be materially different from those reflected in the forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, among others, risks related to: factors that could increase the Company’s cash requirements or expenses beyond its expectations and any factors that could adversely affect its profitability, whether the milestone thresholds can be achieved, delays or difficulties in commencing, enrolling or completing clinical studies, the potential that results from clinical or non-clinical studies indicate the Company’s product candidates or product are unsafe, ineffective or not differentiated, risks of decisions from regulatory authorities that are unfavorable to the Company, dependence on third parties to conduct clinical studies, delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with or relying on third parties to discover, develop, manufacture and commercialize products, and risks associated with establishing and maintaining sales, marketing and distribution capabilities with appropriate technical expertise and supporting infrastructure, the ability of the Company to protect and to enforce its intellectual property rights, volatility and fluctuations in the trading price and volume of the Company’s shares, and general economic and market conditions. Other risks affecting the Company are in the Company’s Form 10-Q filed with the SEC on August 13, 2025, and other periodic reports filed with the SEC. In addition to the risks described above and in Theravance Biopharma’s filings with the SEC, other unknown or unpredictable factors also could affect Theravance Biopharma’s results. No forward-looking statements can be guaranteed, and actual results may differ materially from such statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Theravance Biopharma assumes no obligation to update its forward-looking statements on account of new information, future events or otherwise, except as required by law.Contact:
[email protected]
650-808-40451 Freeman R, et al. Precision therapy with ampreloxetine for neurogenic orthostatic hypotension in multiple system atrophy. MedRxiv. https://www.medrxiv.org/content/10.1101/2025.08.12.25332833v1.
2https://medlineplus.gov/genetics/condition/multiple-system-atrophy/
3 UCSD Neurological Institute (25K-75K, with ~10K new cases per year); NIH National Institute of Neurological Disorders and Stroke (15K-50K).
4 Delveinsight MSA Market Forecast (2023); Symptoms associated with orthostatic hypotension in pure autonomic failure and multiple systems atrophy, CJ Mathias (1999).
5 Data on file. MSA Natural History Statistics, NYU September 2019.SOURCE Theravance Biopharma, Inc.
Continue Reading