Pebble is officially back in the App Store and on Google Play.
Earlier this month the Pebble app returned to iOS and Android with support not just for the new generation of Pebble smartwatches, but also the…

Pebble is officially back in the App Store and on Google Play.
Earlier this month the Pebble app returned to iOS and Android with support not just for the new generation of Pebble smartwatches, but also the…


A superb start from Charles Leclerc lifted him from P3 to P2 in Austin – and a masterclass in defensive driving kept the Ferrari man there for much of the afternoon, despite the best efforts of a marauding Lando Norris.
Ultimately Leclerc had to…

Kylian Mbappé continued his rampant form at the start of the LaLiga season to lead Real Madrid to a 1-0 victory on Sunday and retake top spot in the table.
The game hinged on a bizarre turn of events in the second half when Getafe’s Allan Nyom…

Max Verstappen has claimed a dominant victory at the United States Grand Prix, the Red Bull driver converting pole position into a commanding win while Lando Norris beat Charles Leclerc to second place in a thrilling duel between the pair.
It had…
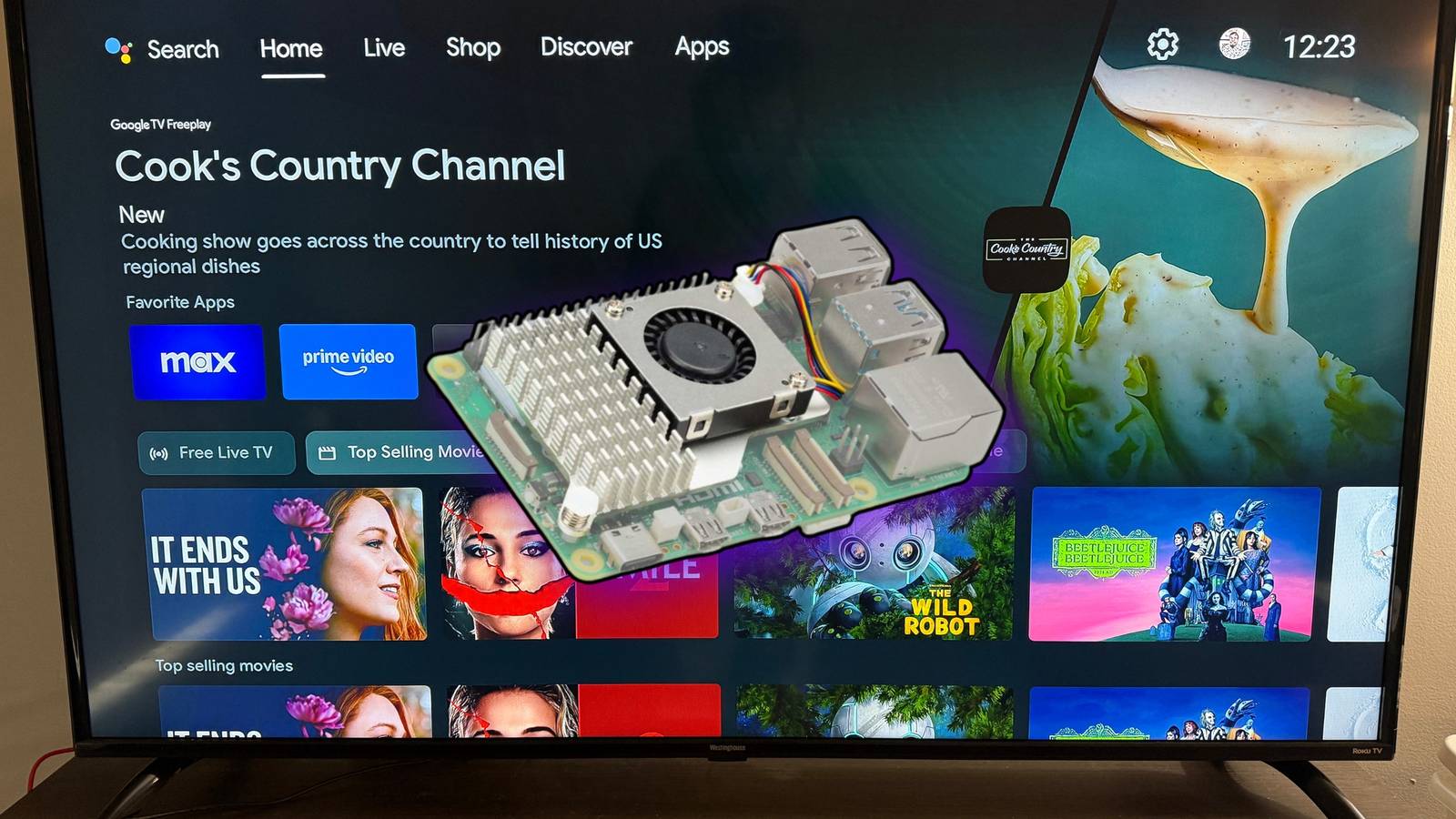
Chromecast was revolutionary when it launched because it turned your normal TV into a system capable of running multiple streaming apps. Even a smart TV that dropped support for apps could gain a new life with a Chromecast. However, there have…

Trodelvy (sacituzumab govitecan) led to a 51% reduction in the risk of progression or death compared with chemotherapy in patients with nonsquamous epidermal growth factor receptor–mutated non–small cell lung cancer that developed epidermal growth factor receptor tyrosine kinase inhibitor resistance, according to phase 3 results of the OptiTROP-Lung04 study presented during the European Society of Medical Oncology Congress 2025.
Results showed that, at a median follow-up of 18.9 months, the median progression-free survival assessed by blinded independent central review was 8.3 months with Trodelvy and 4.3 months with chemotherapy. The 12-month progression-free survival rates were 32% and 8%, respectively. The benefit with Trodelvy was observed across all prespecified subgroups.
The investigator-assessed median progression-free survival was 8.4 months with Trodelvy and 4.8 months with chemotherapy. The 12-month rates were 35% and 11%, respectively.
“Trodelvy demonstrated statistically significant and clinically meaningful improvements in progression-free and overall survival compared to platinum-based chemotherapy,” lead study author Dr. Li Zhang, professor of medical oncology at Sun Yat-sen University Cancer Center in Guangzhou, China, said in an oral presentation of the data. “The results of the OptiTROP-Lung04 study support Trodelvy as a promising new treatment option for patients with EGFR-mutated non–small cell lung cancer with epidermal growth factor receptor tyrosine kinase inhibitor resistance.”
Trodelvy is a TROP2 antibody-drug conjugate with a unique biofunctional linker that maximizes delivery of a belotecan-derivative topoisomerase I inhibitor payload to tumor cells. TROP2 is highly expressed in patients with EGFR-mutated non–small cell lung cancer, and preclinical data have shown that Trodelvy internalization and uptake are enhanced by EGFR mutations.
Progression-free survival (PFS): time during and after treatment that a patient lives without cancer growing or spreading.
Overall survival (OS): time from treatment start or diagnosis until death from any cause.
Objective response rate (ORR): percentage of patients whose cancer shrinks or disappears after treatment.
Disease control rate (DCR): percentage of patients whose cancer shrinks, disappears, or remains stable after treatment.
Duration of response (DOR): length of time a treatment keeps cancer under control after it first responds.
Investigator-assessed PFS: progression-free survival measured by the trial’s treating investigators rather than an independent review.
Current standard options for patients who relapse on third-generation EGFR tyrosine kinase inhibitors remain platinum-based chemotherapy, but more options are needed.
In the multicenter, open-label, phase 3 OptiTROP-Lung04 trial, 376 patients with nonsquamous stage 3B/3C or 4 non–small cell lung cancer with EGFR-sensitive mutations were randomly assigned 1:1 to receive Trodelvy at five milligrams per kilogram intravenously every two weeks or Alimta at 500 milligrams per square meter plus carboplatin area under the curve 5 or cisplatin at 75 milligrams per square meter every three weeks for up to four cycles, followed by Alimta maintenance at 500 milligrams per square meter every three weeks. Treatment was given until disease progression, intolerable toxicity, or patient request to discontinue therapy.
To be eligible for enrollment, patients needed to have an Eastern Cooperative Oncology Group performance status of zero or one and progression after third-generation tyrosine kinase inhibitor therapy or progression after first- or second-generation tyrosine kinase inhibitors with T790M-negative mutations.
Stratification factors included prior EGFR tyrosine kinase inhibitor therapy (third-generation in frontline versus second line versus no third-generation) or brain metastases (yes versus no).
The primary end point was progression-free survival assessed by blinded independent central review; secondary end points were overall survival, investigator-assessed progression-free survival, objective response rate, disease control rate, duration of response, and safety.
A total 148 patients on Trodelvy discontinued treatment due to disease progression (125 patients), patient or guardian withdrawal (12 patients), death (6 patients), side effects (2 patients), or other (3 patients). In the chemotherapy arm, 179 patients discontinued treatment due to disease progression (140 patients), patient/guardian withdrawal (16 patients), death (9 patients), side effects (5 patients), protocol deviation (2 patients), or other (7 patients). A total 69 and 102 patients in each arm, respectively, discontinued from the study due to death (67 and 101 patients) or were lost to follow-up (2 and 1 patients).
Patient baseline characteristics were generally well balanced between the Trodelvy (188 patients) and chemotherapy arms (188 patients). The median age was 60 years and 59 years, and 31% and 27% were at least 65 years. Most had a performance status of 1 (81% and 77%), had no smoking history (77% and 72%), had stage 4 disease (97% and 98%), and at least three metastatic sites (68% and 67%). A total 18% and 19% had brain metastases, and 13% and 18% had liver metastases. The majority in each arm had exon 19 deletions (56% and 63%), had unknown T790M mutation status (59% and 60%), and received a prior third-generation EGFR tyrosine kinase inhibitor in the frontline setting (63% and 62%).
The interim analysis showed that for Trodelvy, the median overall survival was not reached compared with 17.4 months with chemotherapy, leading to a 40% reduction in the risk of death. The 18-month overall survival rates were 66% and 48%, respectively. Overall survival was improved with Trodelvy across all prespecified subgroups.
Dr. Zhang also reported on subsequent anticancer treatment from the trial; 72% of patients on Trodelvy and 86% of those on chemotherapy received at least one subsequent treatment. In the Trodelvy and chemotherapy groups, respectively, these included chemotherapy (42%; 54%), specifically Alimta-based chemotherapy (37%; 13%), an EGFR tyrosine kinase inhibitor (43%; 40%), an anti-angiogenic agent (35%; 48%), immunotherapy (17%; 25%), or an antibody-drug conjugate (1%; 20%).
When assessed via blinded independent central review, the objective response rate with Trodelvy was 61% compared with 43% with chemotherapy; the disease control rate was 87% and 80%, respectively. The median duration of response was 8.3 months with Trodelvy and 4.2 months with chemotherapy; the 12-month rates were 36% versus 8%, respectively.
Regarding safety, treatment-related side effects occurred in 100% and 98% of Trodelvy– and chemotherapy-treated patients, respectively. Grade 3 or higher side effects occurred in 58% and 54% of patients, and serious side effects occurred in 9% and 18% of patients, respectively. Side effects that led to dose reductions and interruptions occurred in 30% and 37% of patients on Trodelvy; there were no side effects that led to discontinuation or death. In the chemotherapy arm, these rates were 23% and 33%; one patient each experienced side effects leading to discontinuation or death.
The median duration of exposure was 9.6 months with Trodelvy and 4.9 months with chemotherapy. The most common side effects in both arms were hematologic; Trodelvy had higher incidence of stomatitis that were mostly grade 1 or 2 (any-grade 62%; grade ≥3 5%). Ocular surface toxicities also occurred in 10% of patients on Trodelvy, all grade 1 or 2. No cases of interstitial lung disease or pneumonitis were reported on the Trodelvy arm.
Phase 3 trials are currently exploring Trodelvy alone and in combination with Tagrisso in patients with EGFR-mutant non–small cell lung cancer.
For more news on cancer updates, research and education,

Travis Kelce showed up in a chic fit ahead of the Las Vegas Raiders-Kansas City Chiefs game on Sunday. The NFL shared a clip of the tight end’s look on X.
Kelce, Taylor Swift’s fiancé, wore black pants, a gray undershirt and…

When the NFL, Roc Nation, and Apple Music announced that Bad Bunny would be the 2026 Super Bowl halftime performer, the noise started almost immediately. On one side, there were cheers and excitement: His fans recognized the historic nature of…