His mother, Karen, shares the sentiment.
“I’m so happy I can finally show him around my hometown,” she said. “He gets to see where I grew up, where I slept. My parents are hosting the whole gymnastics team for a special dinner. It’s…

His mother, Karen, shares the sentiment.
“I’m so happy I can finally show him around my hometown,” she said. “He gets to see where I grew up, where I slept. My parents are hosting the whole gymnastics team for a special dinner. It’s…
– Late-Breaking ASCENT-03 Data Simultaneously Presented at ESMO 2025 and Published in The New England Journal of Medicine –
– With Two Positive Phase 3 Trials, Trodelvy Has Potential as the First and Only ADC to be a Backbone Standard of Care for All First-Line Metastatic TNBC Patients Regardless of PD-L1 Status –
FOSTER CITY, Calif.–(BUSINESS WIRE)–
Gilead Sciences, Inc. (Nasdaq: GILD) today shared positive data from the Phase 3 ASCENT-03 study demonstrating a highly statistically significant and clinically meaningful improvement in progression-free survival (PFS) for Trodelvy® (sacituzumab govitecan-hziy) compared to chemotherapy as first-line treatment in patients with metastatic triple-negative breast cancer (TNBC) who are not candidates for PD-1/PD-L1 inhibitors. These findings will be presented today during a late-breaking oral session at the 2025 European Society for Medical Oncology (ESMO) Congress (Abstract #LBA20) and simultaneously published in The New England Journal of Medicine.
ASCENT-03 successfully met its primary endpoint of PFS with a 38% reduced risk of disease progression or death for Trodelvy versus chemotherapy (HR: 0.62; p<0.0001). Median PFS with Trodelvy was 9.7 months versus 6.9 months for chemotherapy. The PFS results for Trodelvy versus chemotherapy were consistent across prespecified subgroups, including among patients with poorer prognosis (such as those whose cancer recurred less than one year after treatment in the curative setting) and regardless of chemotherapy chosen.
“Patients with metastatic TNBC who are ineligible for immunotherapy face an especially poor prognosis, with limited treatment options and fast disease progression,” said Dr. Javier Cortés, Head of the International Breast Cancer Center in Spain and principal investigator of the ASCENT-03 study. “The ability of sacituzumab govitecan to significantly delay death and progression could represent the first major treatment advance for this patient population in the 20 years since TNBC was defined, marking an historic shift and establishing a potential new standard of care.”
The objective response rate (ORR) was 48% with Trodelvy and 46% with chemotherapy. Median duration of response (DOR) was substantially longer with Trodelvy. Among patients with confirmed complete or partial responses, DOR for Trodelvy was 12.2 months compared to 7.2 months with chemotherapy.
Overall survival (OS) data were not mature at the time of PFS primary analysis. Gilead will continue to monitor OS outcomes, with ongoing patient follow-up and further analysis planned.
These results complement the recent presentation of ASCENT-04/KEYNOTE-D19, which showed a significant PFS benefit for Trodelvy plus Keytruda® (pembrolizumab) in PD-L1+ first-line metastatic TNBC. Gilead is engaging with the U.S. Food and Drug Administration and other global regulators regarding both data sets.
“ASCENT-03 is the second Phase 3 trial with a Trodelvy-based regimen to show superior progression-free survival over chemotherapy in first-line metastatic TNBC, highlighting its potential to improve outcomes for patients with limited treatment options,” said Dietmar Berger, MD, PhD, Chief Medical Officer, Gilead Sciences. “With these potentially practice-changing results, Trodelvy is poised to transform the first-line metastatic TNBC treatment landscape, offering a much-needed alternative to chemotherapy.”
Summary of Key ASCENT-03 Results
|
Efficacy: Intent-to-Treat Population |
Trodelvy (n=279) |
Chemotherapy (n=279) |
|
Median PFS |
9.7 months (95% CI: 8.1-11.1) |
6.9 months (95% CI: 5.6-8.2) |
|
PFS hazard ratio and p-value |
0.62 (95% CI: 0.50-0.77); p<0.0001 |
|
|
ORR |
48% (95% CI: 42-54) |
46% (95% CI: 40-52) |
|
DOR |
12.2 months (95% CI: 9.7-13.8) |
7.2 months (95% CI: 5.7-8.4) |
The safety profile of Trodelvy in the ASCENT-03 study was consistent with prior studies, and no new safety signals were identified in this patient population. The most frequent grade ≥3 treatment-emergent adverse events were neutropenia (43%) and diarrhea (9%) with Trodelvy and neutropenia (41%) and anemia (16%) with chemotherapy. Fewer patients discontinued treatment due to adverse events on Trodelvy than with chemotherapy (4% vs. 12%).
Healthcare professionals have well-established experience with Trodelvy, with more than 60,000 breast cancer patients treated across 50+ countries over the past five years. It remains the only Trop-2-directed antibody-drug conjugate (ADC) to demonstrate meaningful survival benefits in both 2L+ metastatic TNBC and pre-treated HR+/HER2- metastatic breast cancer. It is also the only ADC with four positive Phase 3 trials in HER2- mBC (IHC 0, IHC 1+, or IHC 2+/ISH–).
The use of Trodelvy plus Keytruda in patients with first-line PD-L1+ metastatic TNBC and Trodelvy as monotherapy in patients with first-line metastatic TNBC who are not candidates for PD-1/PD-L1 inhibitors are investigational, and the safety and efficacy of these uses have not been established.
KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
About Triple-Negative Breast Cancer (In Patients Who Are Not Candidates for PD-1/PD-L1 inhibitors)
Triple-negative breast cancer (TNBC) is the most aggressive type of breast cancer and has historically been difficult to treat, accounting for approximately 15% of all breast cancers. TNBC disproportionately impacts younger, pre-menopausal as well as Black and Hispanic women. TNBC cells do not have estrogen and progesterone receptors and have limited HER2. Due to the nature of TNBC, treatment options are extremely limited compared with other breast cancer types. TNBC has a higher chance of recurrence and metastases than other breast cancer types. The average time to metastatic recurrence for TNBC is approximately 2.6 years compared with 5 years for other breast cancers, and the relative five-year survival rate is much lower. Among women with metastatic TNBC, the five-year survival rate is 12%, compared with 28% for those with other types of metastatic breast cancer.
Chemotherapy remains the mainstay of treatment in first-line metastatic TNBC patients who are not candidates for PD-1/PD-L1 inhibitors, and the need to improve outcomes continues to be high as there has not been a clinically meaningful advance for this patient population in over 20 years. In metastatic TNBC overall, ~50% of patients do not receive treatment beyond the first-line setting, demonstrating a need for additional effective earlier-line treatment options.
About the ASCENT-03 Study
The ASCENT-03 study is a global, open-label, randomized Phase 3 trial evaluating the efficacy and safety of Trodelvy compared with treatment of physician’s choice in patients with previously untreated, locally advanced, inoperable, or metastatic triple-negative breast cancer whose tumors do not express PD-L1, or who are PD-L1 positive and previously treated with a PD-(L)1 inhibitor in the curative setting. 558 patients were enrolled across multiple study sites worldwide.
Patients were randomized 1:1 to receive either Trodelvy (10 mg/kg intravenously on Days 1 and 8 of a 21-day cycle) or treatment of physician’s choice, which included gemcitabine plus carboplatin, paclitaxel, or nab-paclitaxel. Treatment continued until blinded independent central review (BICR)-verified disease progression or unacceptable toxicity. Patients randomized to chemotherapy were eligible to cross over to Trodelvy upon disease progression.
The primary endpoint of the study is progression-free survival (PFS) as assessed by BICR according to RECIST v1.1. Secondary endpoints include overall survival (OS), objective response rate (ORR), duration of response (DOR), time to onset of response (TTR), patient-reported outcomes (PROs) and safety.
More information about ASCENT-03 is available at ClinicalTrials.gov: NCT05382299.
About Trodelvy
Trodelvy (sacituzumab govitecan-hziy) is a first-in-class Trop-2-directed antibody-drug conjugate. Trop-2 is a cell surface antigen highly expressed in multiple tumor types, including in more than 90% of breast and lung cancers. Trodelvy is intentionally designed with a proprietary hydrolyzable linker attached to SN-38, a topoisomerase I inhibitor payload. This unique combination delivers potent activity to both Trop-2 expressing cells and the tumor microenvironment through a bystander effect.
Trodelvy is currently approved in more than 50 countries for second-line or later metastatic triple-negative breast cancer (TNBC) patients and in more than 40 countries for certain patients with pre-treated HR+/HER2- metastatic breast cancer.
Trodelvy is currently being evaluated in multiple ongoing Phase 3 trials across a range of tumor types with high Trop-2 expression. These studies with Trodelvy, both in monotherapy and in combination with pembrolizumab, involve earlier lines of treatment for TNBC and HR+/HER2- breast cancer—including in curative settings—as well as in lung and gynecologic cancers, where previous proof-of-concept studies have demonstrated clinical activity.
INDICATIONS
TRODELVY® (sacituzumab govitecan-hziy) is a Trop-2-directed antibody and topoisomerase inhibitor conjugate indicated for the treatment of adult patients with:
IMPORTANT SAFETY INFORMATION
BOXED WARNING: NEUTROPENIA AND DIARRHEA
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
Neutropenia: Severe, life-threatening, or fatal neutropenia can occur as early as the first cycle of treatment and may require dose modification. Neutropenia occurred in 64% of patients treated with TRODELVY. Grade 3-4 neutropenia occurred in 49% of patients. Febrile neutropenia occurred in 6%. Neutropenic colitis occurred in 1.4%. Primary prophylaxis with G-CSF is recommended starting in the first cycle of treatment in all patients at increased risk of febrile neutropenia, including older patients, patients with previous neutropenia, poor performance status, organ dysfunction, or multiple comorbidities. Monitor absolute neutrophil count (ANC) during treatment. Withhold TRODELVY for ANC below 1500/mm3 on Day 1 of any cycle or below 1000/mm3 on Day 8 of any cycle. Withhold TRODELVY for neutropenic fever. Treat neutropenia with G-CSF and administer prophylaxis in subsequent cycles as clinically indicated or indicated in Table 2 of USPI.
Diarrhea: Diarrhea occurred in 64% of all patients treated with TRODELVY. Grade 3-4 diarrhea occurred in 11% of patients. One patient had intestinal perforation following diarrhea. Diarrhea that led to dehydration and subsequent acute kidney injury occurred in 0.7% of all patients. Withhold TRODELVY for Grade 3-4 diarrhea and resume when resolved to ≤ Grade 1. At onset, evaluate for infectious causes and if negative, promptly initiate loperamide, 4 mg initially followed by 2 mg with every episode of diarrhea for a maximum of 16 mg daily. Discontinue loperamide 12 hours after diarrhea resolves. Additional supportive measures (e.g., fluid and electrolyte substitution) may also be employed as clinically indicated. Patients who exhibit an excessive cholinergic response to treatment can receive appropriate premedication (e.g., atropine) for subsequent treatments.
Hypersensitivity and Infusion-Related Reactions: TRODELVY can cause serious hypersensitivity reactions including life-threatening anaphylactic reactions. Severe signs and symptoms included cardiac arrest, hypotension, wheezing, angioedema, swelling, pneumonitis, and skin reactions. Hypersensitivity reactions within 24 hours of dosing occurred in 35% of patients. Grade 3-4 hypersensitivity occurred in 2% of patients. The incidence of hypersensitivity reactions leading to permanent discontinuation of TRODELVY was 0.2%. The incidence of anaphylactic reactions was 0.2%. Pre-infusion medication is recommended. Have medications and emergency equipment to treat such reactions available for immediate use. Observe patients closely for hypersensitivity and infusion-related reactions during each infusion and for at least 30 minutes after completion of each infusion. Permanently discontinue TRODELVY for Grade 4 infusion-related reactions.
Nausea and Vomiting: TRODELVY is emetogenic and can cause severe nausea and vomiting. Nausea occurred in 64% of all patients treated with TRODELVY and Grade 3-4 nausea occurred in 3% of these patients. Vomiting occurred in 35% of patients and Grade 3-4 vomiting occurred in 2% of these patients. Premedicate with a two or three drug combination regimen (e.g., dexamethasone with either a 5-HT3 receptor antagonist or an NK1 receptor antagonist as well as other drugs as indicated) for prevention of chemotherapy-induced nausea and vomiting (CINV). Withhold TRODELVY doses for Grade 3 nausea or Grade 3-4 vomiting and resume with additional supportive measures when resolved to Grade ≤ 1. Additional antiemetics and other supportive measures may also be employed as clinically indicated. All patients should be given take-home medications with clear instructions for prevention and treatment of nausea and vomiting.
Increased Risk of Adverse Reactions in Patients with Reduced UGT1A1 Activity: Patients homozygous for the uridine diphosphate-glucuronosyl transferase 1A1 (UGT1A1)*28 allele are at increased risk for neutropenia, febrile neutropenia, and anemia and may be at increased risk for other adverse reactions with TRODELVY. The incidence of Grade 3-4 neutropenia was 58% in patients homozygous for the UGT1A1*28, 49% in patients heterozygous for the UGT1A1*28 allele, and 43% in patients homozygous for the wild-type allele. The incidence of Grade 3-4 anemia was 21% in patients homozygous for the UGT1A1*28 allele, 10% in patients heterozygous for the UGT1A1*28 allele, and 9% in patients homozygous for the wild-type allele. Closely monitor patients with known reduced UGT1A1 activity for adverse reactions. Withhold or permanently discontinue TRODELVY based on clinical assessment of the onset, duration and severity of the observed adverse reactions in patients with evidence of acute early-onset or unusually severe adverse reactions, which may indicate reduced UGT1A1 function.
Embryo-Fetal Toxicity: Based on its mechanism of action, TRODELVY can cause teratogenicity and/or embryo-fetal lethality when administered to a pregnant woman. TRODELVY contains a genotoxic component, SN-38, and targets rapidly dividing cells. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with TRODELVY and for 6 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with TRODELVY and for 3 months after the last dose.
ADVERSE REACTIONS
In the pooled safety population, the most common (≥ 25%) adverse reactions including laboratory abnormalities were decreased leukocyte count (84%), decreased neutrophil count (75%), decreased hemoglobin (69%), diarrhea (64%), nausea (64%), decreased lymphocyte count (63%), fatigue (51%), alopecia (45%), constipation (37%), increased glucose (37%), decreased albumin (35%), vomiting (35%), decreased appetite (30%), decreased creatinine clearance (28%), increased alkaline phosphatase (28%), decreased magnesium (27%), decreased potassium (26%), and decreased sodium (26%).
In the ASCENT study (locally advanced or metastatic triple-negative breast cancer), the most common adverse reactions (incidence ≥25%) were fatigue, diarrhea, nausea, alopecia, constipation, vomiting, abdominal pain, and decreased appetite. The most frequent serious adverse reactions (SAR) (>1%) were neutropenia (7%), diarrhea (4%), and pneumonia (3%). SAR were reported in 27% of patients, and 5% discontinued therapy due to adverse reactions. The most common Grade 3-4 lab abnormalities (incidence ≥25%) in the ASCENT study were reduced neutrophils, leukocytes, and lymphocytes.
In the TROPiCS-02 study (locally advanced or metastatic HR-positive, HER2-negative breast cancer), the most common adverse reactions (incidence ≥25%) were diarrhea, fatigue, nausea, alopecia, and constipation. The most frequent serious adverse reactions (SAR) (>1%) were diarrhea (5%), febrile neutropenia (4%), neutropenia (3%), abdominal pain, colitis, neutropenic colitis, pneumonia, and vomiting (each 2%). SAR were reported in 28% of patients, and 6% discontinued therapy due to adverse reactions. The most common Grade 3-4 lab abnormalities (incidence ≥25%) in the TROPiCS-02 study were reduced neutrophils and leukocytes.
DRUG INTERACTIONS
UGT1A1 Inhibitors: Concomitant administration of TRODELVY with inhibitors of UGT1A1 may increase the incidence of adverse reactions due to potential increase in systemic exposure to SN-38. Avoid administering UGT1A1 inhibitors with TRODELVY.
UGT1A1 Inducers: Exposure to SN-38 may be reduced in patients concomitantly receiving UGT1A1 enzyme inducers. Avoid administering UGT1A1 inducers with TRODELVY.
Please see full Prescribing Information, including BOXED WARNING.
About Gilead and Kite Oncology
Gilead and Kite Oncology are working to transform how cancer is treated. We are innovating with next-generation therapies, combinations and technologies to deliver improved outcomes for people with cancer. We are purposefully building our oncology portfolio and pipeline to address the greatest gaps in care. From antibody-drug conjugate technologies and small molecules to cell therapy-based approaches, we are creating new possibilities for people with cancer.
About Gilead Sciences
Gilead Sciences, Inc. is a biopharmaceutical company that has pursued and achieved breakthroughs in medicine for more than three decades, with the goal of creating a healthier world for all people. The company is committed to advancing innovative medicines to prevent and treat life-threatening diseases, including HIV, viral hepatitis, COVID-19, cancer and inflammation. In 2025, Gilead announced a planned $32 billion investment to further strengthen its U.S. footprint to power the next era of discovery, job creation and public health preparedness – while continuing to invest globally to ensure patients everywhere benefit from its scientific innovation. Gilead operates in more than 35 countries worldwide, with headquarters in Foster City, Calif.
Forward-Looking Statements
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks, uncertainties and other factors, including Gilead’s ability to initiate, progress or complete clinical trials or studies within currently anticipated timelines or at all, and the possibility of unfavorable results from ongoing and additional clinical trials or studies, including those involving Trodelvy (such as ASCENT-03); uncertainties relating to regulatory applications and related filing and approval timelines, including potential applications for programs and/or indications currently under evaluation; the possibility that Gilead may make a strategic decision to discontinue development of these programs and, as a result, these programs may never be successfully commercialized for the indications currently under evaluation; and any assumptions underlying any of the foregoing. These and other risks, uncertainties and factors are described in detail in Gilead’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2025, as filed with the U.S. Securities and Exchange Commission. These risks, uncertainties and other factors could cause actual results to differ materially from those referred to in the forward-looking statements. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. The reader is cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties and is cautioned not to place undue reliance on these forward-looking statements. All forward-looking statements are based on information currently available to Gilead, and Gilead assumes no obligation and disclaims any intent to update any such forward-looking statements.
Trodelvy, Gilead and the Gilead logo are trademarks of Gilead Sciences, Inc., or its related companies.
U.S. Prescribing Information for Trodelvy, including BOXED WARNING, is available at www.gilead.com.
For more information about Gilead, please visit the company’s website at www.gilead.com, follow Gilead on X/Twitter (@Gilead Sciences) and LinkedIn (@Gilead-Sciences).
Source: Gilead Sciences, Inc.

Club members are hoping the local community might express interest in taking over the site, perhaps using it as a community hub for events and film screenings.
That is why they decided to hold an EGM rather than wait for the club’s usual AGM next…

Match Report
Western Bulldogs thump Gold Coast by 66 points in Mackay.
It may sound unbelievable, but new research suggests that instead of being featureless, dark matter could actually behave like a cosmic superfluid, forming swirling vortex lines and stable rotating cores known as solitons inside…


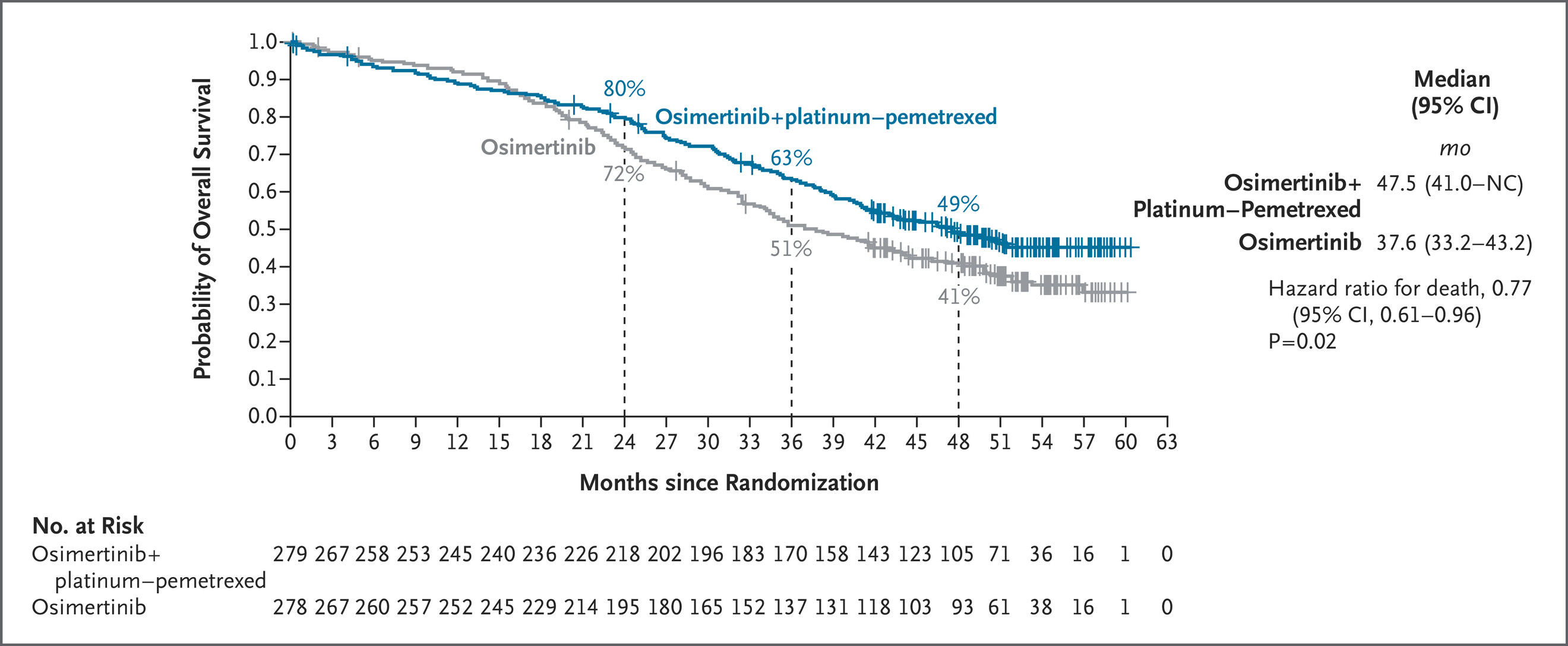
The Phase 3 FLAURA2 trial (NCT04035486) continues to strengthen the role of combination therapy in the first-line management of EGFR-mutated (EGFRm) advanced non–small cell lung cancer (NSCLC). At ESMO 2025, investigators reported the final overall survival (OS) analysis, showing that osimertinib (osi) plus platinum–pemetrexed chemotherapy (CTx) achieved a statistically significant and clinically meaningful OS improvement compared with osimertinib monotherapy.
This milestone confirms the combination as a first-line standard of care in EGFRm NSCLC, offering durable efficacy across key subgroups, including those with traditionally poor prognostic features such as CNS metastases, L858R mutations, plasma-detectable EGFRm, and altered TP53.
The FLAURA2 trial enrolled 557 patients with EGFRm advanced NSCLC, who were randomized 1:1 to receive osimertinib plus chemotherapy (n=279) or osimertinib alone (n=278). The chemotherapy regimen consisted of platinum–pemetrexed, administered concurrently with osimertinib.
Baseline CNS imaging (CT or MRI) was mandatory to identify central nervous system metastases. EGFR mutation subtypes (Ex19del or L858R) were confirmed through central or locally approved assays. Plasma EGFR mutation detection was evaluated via droplet digital PCR (Biodesix), while TP53 mutation status was determined using FoundationOne CDx.
OS outcomes were analyzed using an unstratified Cox proportional hazards model, with subgroup analyses conducted based on baseline prognostic factors.
A total of 557 patients were included: 279 in the osimertinib + CTx arm and 278 in the osimertinib monotherapy arm. The overall hazard ratio (HR) for OS was 0.77 (95% CI 0.61–0.96; p=0.02), corresponding to a 23% reduction in risk of death with the combination regimen.
In patients with baseline CNS metastases, median OS reached 40.9 months (95% CI 35.2–46.6) with osimertinib + CTx versus 29.7 months (95% CI 25.6–35.8) with monotherapy (HR 0.72; 95% CI 0.52–0.99).
Both EGFRm subgroups derived benefit.
Patients with detectable plasma EGFRm achieved median OS 38.4 vs 32.5 months (HR 0.79; 95% CI 0.60–1.03), while those with undetectable mutations had not reached median OS in either arm.
Among patients with altered TP53, OS improved to 51.1 months vs 43.1 months (HR 0.71; 95% CI 0.40–1.27), showing consistent benefit across molecular profiles.
No new safety signals were reported, confirming that the addition of chemotherapy did not compromise the established tolerability of osimertinib.
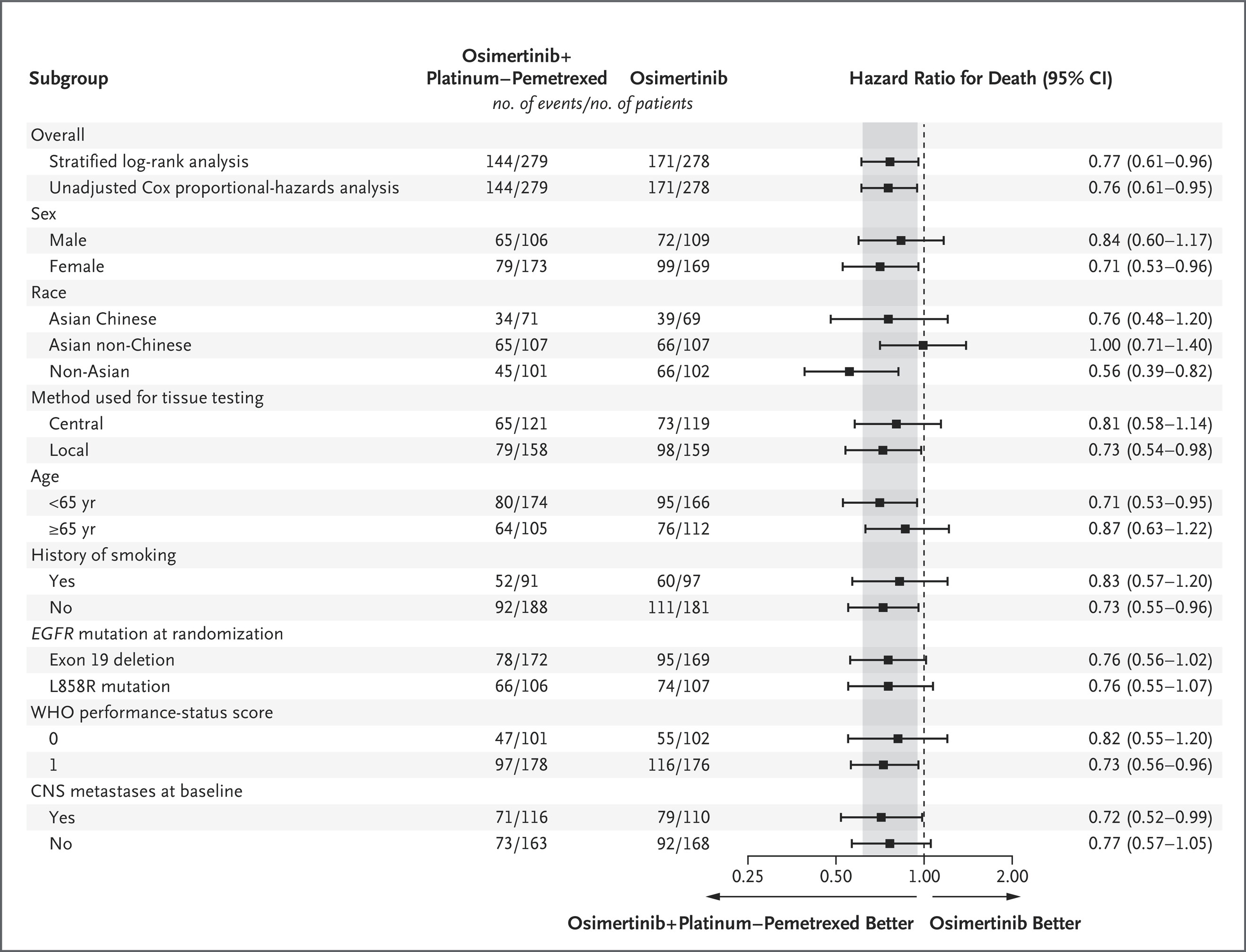
The survival improvement observed with osimertinib plus chemotherapy extended across every prognostic subgroup examined. The HRs for OS within each category closely mirrored those of the overall intent-to-treat (ITT) population, highlighting the robustness and reproducibility of the benefit.
Particularly noteworthy were the gains among patients with CNS metastases and L858R mutation, two subsets traditionally associated with poorer prognosis. These data underline the capacity of the combination regimen to overcome key resistance mechanisms and delay disease progression.
The long-term follow-up from the FLAURA2 trial at ESMO 2025 provides the most comprehensive survival dataset to date for EGFR-mutated NSCLC treated with first-line osimertinib plus chemotherapy. With several subgroups not yet reaching median OS, the sustained separation of survival curves indicates a persistent and cumulative treatment effect extending well beyond the chemotherapy phase.
Investigators emphasized that the survival curves for osimertinib + CTx began to diverge early—within the first year of treatment—and remained separated throughout follow-up, suggesting that early dual blockade of EGFR signaling and microenvironmental suppression via chemotherapy offers durable control of micrometastatic disease.
The analysis also highlights that median OS was not reached in multiple subgroups, demonstrating an ongoing trend toward improved long-term outcomes. The consistency of benefit across genomic and clinical subgroups—including those with TP53 alterations or detectable circulating tumor DNA—supports the biological rationale that concurrent chemotherapy may help prevent the emergence of resistant clones.
Methodologically, the trial’s unstratified Cox model ensured unbiased subgroup comparisons and confirmed that the treatment effect was independent of baseline risk characteristics. Furthermore, despite crossover and subsequent therapies available post-progression, the OS advantage with osimertinib plus chemotherapy persisted, reinforcing the value of front-line combination therapy over sequential monotherapy strategies.
No late-emerging toxicities or cumulative safety concerns were identified during long-term follow-up, strengthening the safety profile of the regimen. Together, these findings establish osimertinib plus chemotherapy as a durable, well-tolerated, and biologically rational first-line standard of care for patients with EGFR-mutated advanced NSCLC.
Read Full Abstract on ESMO Congress 2025 Website
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD