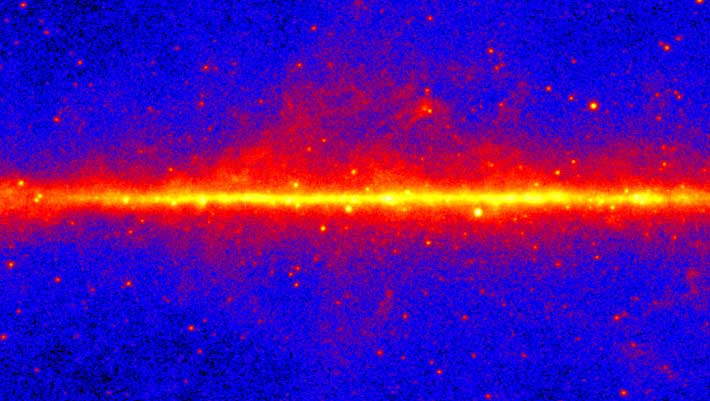
The Galactic Center excess is an unexpected concentration of gamma-rays emerging from the center of our Milky Way Galaxy.
This view shows the entire sky at energies greater than 1 GeV based on five years of data from the LAT instrument on…
The Galactic Center excess is an unexpected concentration of gamma-rays emerging from the center of our Milky Way Galaxy.
This view shows the entire sky at energies greater than 1 GeV based on five years of data from the LAT instrument on…
Adjuvant atezolizumab (Tecentiq) generated a significant improvement in disease-free survival (DFS) and overall survival (OS) compared with placebo in patients with muscle-invasive bladder cancer (MIBC) who were positive for circulating tumor DNA (ctDNA) by serial testing, according to data from the phase 3 IMvigor11 trial (NCT04660344).1
Findings presented at the
Additionally, ctDNA-positive patients in the atezolizumab arm experienced a median OS of 32.8 months (95% CI, 27.7-not evaluable [NE]) compared with 21.1 months (95% CI, 14.7-NE) for those in the placebo arm (HR, 0.59; 95% CI, 0.39-0.90; P = .0131).1
“Serial ctDNA testing is a novel approach in this setting. One could argue it’s more accurate than the radiology [assessments] we’re using, and perhaps we can start looking at these diseases differently, beyond bladder cancer. Instead of telling patients that they’ve got the all clear and then radiologically [have their] cancer return, we can be doing tests looking for MRD and identifying early metastatic disease, bringing a new ctDNA-positive, radiology-negative group of patients,” lead study author Thomas Powles, MBBS, MRCP, MD, said in a presentation of the data.
Powles is a professor of genitourinary oncology, director of the Barts Cancer Centre at St. Bartholomew’s Hospital, and lead for Solid Tumor Research at Queen Mary University in London.
Radical cystectomy with or without neoadjuvant therapy represents a potential curative option for patients with MIBC, but Powles explained that disease recurrence remains a challenge and is associated with a poor prognosis.
Previously reported data from the phase 3 IMvigor10 trial (NCT02450331) showed that adjuvant atezolizumab did not produce DFS or OS benefits vs placebo in patients MIBC who were unselected for ctDNA.3 However, an exploratory analysis from that trial showed that patients who were positive for ctDNA after cystectomy experienced an OS benefit, whereas those with ctDNA-negative disease did not have a survival benefit.1
“[IMvigor10] is an example of one of the big negative trials that we’ve performed in bladder cancer. The group…went back to [IMvigor10] and performed an exploratory analysis, [then] launched a subsequent phase 3 [study (IMvigor11)] based on that,” Powles explained.
Thusly, the IMvigor11 trial sought to further explore the potential role of adjuvant atezolizumab in ctDNA-positive MIBC.
Investigators enrolled patients with MIBC who underwent radical cystectomy within 6 to 24 weeks of screening. Patients needed to have histologically confirmed (y)pT2-T4aN0M0 or (y)pT0-T4aN+M0 urothelial cancer with no evidence of radiographic disease progression. Prior neoadjuvant therapy was allowed, and patients needed to have an ECOG performance status of 0 to 2.
Enrolled patients underwent serial ctDNA testing every 6 weeks and radiographic imaging every 12 weeks, and serial ctDNA testing was continued for any patients who tested negative. The Signatera MRD test was used for ctDNA detection for patients enrolled outside mainland China, and those in China were tested using the BGI MRD test.
Patients who remained ctDNA negative for up to 1 year did not receive any treatment, and surveillance continued with follow-up. Patients who tested positive for ctDNA at any point without evidence of radiographic disease were randomly assigned in a 2:1 ratio to receive atezolizumab at 1680 mg once every 4 weeks for up to 1 year, or matching placebo.
Investigator-assessed DFS served as the trial’s primary end point, and OS was a key secondary end point.
During the study, 379 patients tested positive for ctDNA at any point, and 377 patients remained in persistent ctDNA negativity. In the ctDNA-positive population, 129 patients were excluded dur to investigator-assessed radiographic recurrence (n = 72), independent review–assessed radiographic recurrence, and other (n = 34). Furthermore, 250 ctDNA-positive patients were randomly assigned and included in the efficacy population; the safety population included 248 patients who received treatment with either atezolizumab (n = 165) or placebo (n = 83).
At the data cutoff, 8 patients were ongoing treatment in the experimental arm vs 4 patients in the placebo arm. Ninety-two and 35 patients, respectively, remained in follow-up; 67 and 44 patients, respectively, discontinued the study.
In the ctDNA-negative population, 20 patients were excluded due to no post-baseline assessment (n = 14), insufficient follow-up (n = 5), and no cystectomy (n = 1). Overall, 357 patients were evaluable for efficacy in the ctDNA-negative population; 310 completed or were continuing surveillance. Forty-seven patients discontinue the study due to investigator-assessed radiographic progression (n = 18), patient withdrawal (n = 14), death (n = 11), and other (n = 4).
Within the ctDNA-positive population, the median age was 69 years (range, 42-87) in the atezolizumab arm vs 67 years (range, 44-84) in the placebo arm. Most patients in both groups were male (atezolizumab, 84.4%; placebo, 80.7%), were from Europe (60.5%; 59.0%), had an ECOG performance status of 0 (67.7%; 63.9%), had a PD-L1 expression of less than 5% (64.7%; 63.9%), did not receive neoadjuvant chemotherapy (52.1%; 60.2%), had positive nodal status (57.5%; 57.8%), were less than 20 weeks removed from cystectomy to first positive ctDNA sample (70.1%; 71.1%), and were ctDNA positive on their initial test (59.3%; 59.0%).
The DFS and OS benefits with atezolizumab were consistent across subgroups in the ctDNA-positive population. Specifically, patients who tested positive for ctDNA on their initial test experienced a median investigator-assessed DFS of 8.3 months (95% CI, 6.2-12.6) with atezolizumab (n = 99) vs 4.2 months (95% CI, 2.4-7.7) with placebo (n = 49; HR, 0.62; 95% CI, 0.42-0.91). For patients who tested positive for ctDNA on a subsequent test, the median DFS was 10.5 months (95% CI, 7.1-14.6) with atezolizumab (n = 68) vs 8.3 months (95% CI, 4.2-12.6) with placebo (n = 34; HR, 0.66; 95% CI, 0.40-1.10).
In the ctDNA-positive population, the median OS for patients positive at their first test was 27.7 months (95% CI, 22.0-35.9) with atezolizumab vs 18.2 months (95% CI, 10.9-NE) with placebo (HR, 0.71; 95% CI, 0.43-1.17). The median OS was NE (95% CI, 34.4-NE) with atezolizumab vs 27.4 months (95% CI, 18.1-NE) with placebo in those patients who tested positive for ctDNA after the initial screening (HR, 0.52; 95% CI, 0.24-1.12).
Additionally, ctDNA clearance at cycle 3, day 1, or cycle 5, day 1 occurred in 25.1% of patients in the atezolizumab arm vs 14.5% of patients in the placebo arm.
Disease recurrence occurred in 63.5% of patients in the atezolizumab arm vs 78.3% of patients in the placebo arm; 51.5% and 54.2%, respectively, received follow-up therapy. In the atezolizumab arm, 48.5% of patients completed first-line therapy, 17.4% finished second-line therapy, 4.2% completed third-line therapy, and 1.2% finished fourth-line therapy. These respective rates were 51.8%, 21.7%, 4.8%, and 1.2% in the placebo arm. Subsequent treatments in the experimental arm included chemotherapy at 36.5%, immunotherapy at 15.6%, an antibody-drug conjugate at 19.2%, and other at 6.0%. These respective rates were 31.3%, 31.3%, 19.3%, and 4.8% in the control arm.
In the ctDNA-positive population, any-grade adverse effects (AEs) were reported in 83.6% of patients in the atezolizumab arm vs 85.5% of patients in the placebo arm; the rates of grade 3/4 AEs were 28.5% and 21.7%, respectively. Any-grade treatment-related AEs (TRAEs) occurred at respective rates of 49.1% and 50.6%, and the rates of grade 3/4 TRAEs were 7.3% and 3.6%, respectively. Serious AEs occurred in 26.7% of patients in the experimental arm vs 20.5% of patients in the control arm; the rates of serious TRAEs were 5.5% and 0%, respectively.
AEs led to death in 3.0% of patients in the atezolizumab arm compared with 2.4% of patients in the placebo arm. TRAEs led to death in 1.8% of patients in the experimental arm vs no patients in the placebo arm.
AEs led to treatment discontinuation and treatment interruption in 9.1% and 23.6% of patients in the atezolizumab arm, respectively. These respective rates were 3.6% and 19.3% in the placebo group.
Immune-mediated AEs of any grade were reported in 38.8% of patients in the atezolizumab arm vs 12.0% of patients in the control arm. The respective rates of grade 3/4 immune-mediated AEs were 4.8% and 1.2%. Immune-mediated AEs led to death in 0.6% of patients in the atezolizumab group vs no patients in the control group.
At a median follow-up of 21.8 months for evaluable ctDNA-negative patients who underwent surveillance, the median DFS was NE (95% CI, NE-NE), with 1- and 2-year DFS rates of 95.4% and 88.4%, respectively.The median OS was also NE (95% CI, NE-NE), with 1- and 2-year OS rates of 100% and 97.1%, respectively.
Perplexity has officially launched Comet, its new AI-powered browser, to all users globally. The company confirmed that the browser can now be downloaded for free at perplexity.ai/comet, opening access to what was previously limited to its…
This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
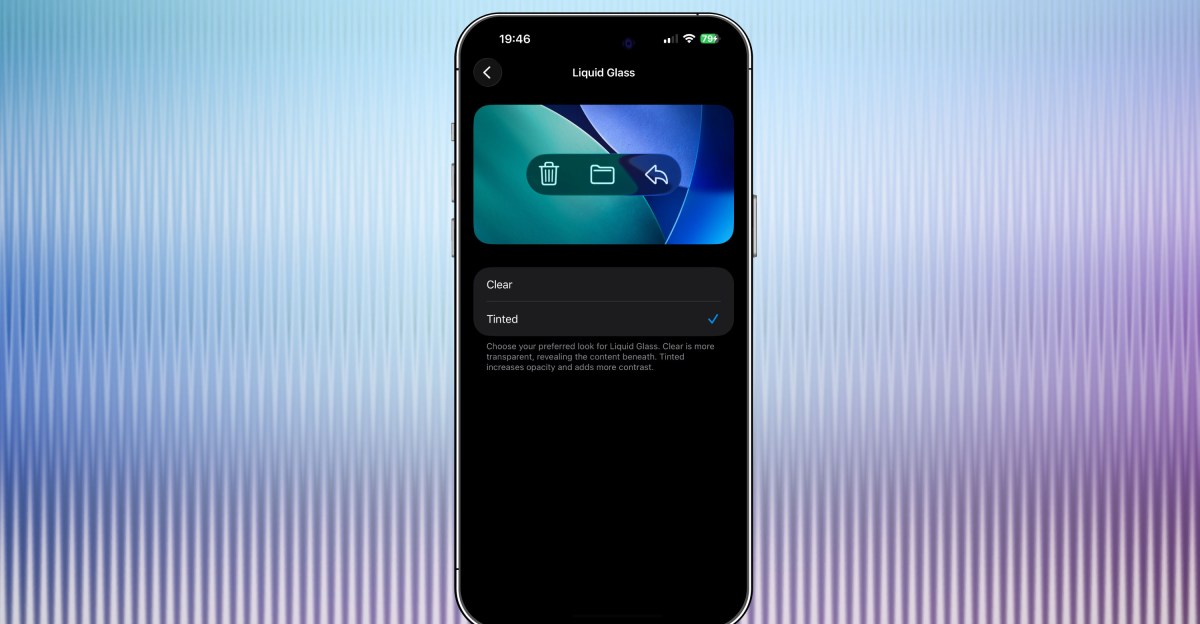
Apple’s latest iOS 26.1 beta has a new option that lets you tint Liquid Glass elements of iOS 26 so that they are more opaque. Since announcing Liquid Glass at WWDC this year, Apple has tweaked exactly how glassy Liquid Glass is — early on,…

The 76ers head to Boston to take on the Celtics for their 2025-26 season tip-off.
The opening week of the NBA season is finally here, and you can catch some of the best matchups exclusively on NBA League Pass.
Oct. 22: Sixers at Celtics (7:30…

Survival disparities by health insurance and race among patients with newly diagnosed breast cancer have widened in the US, according to findings from an American Cancer Study (ACS)–led study presented at the 2025 American Society of Clinical…

During each event period, you can tackle a special Emerald Rush challenge that shakes up the usual formula by setting some fixed conditions, such as the Emerald Perks you can obtain from fossils and Banandium Gems. You’ll still be able to…

Lorenz Hart, lyricist who was the longtime toast of Broadway, died at age 48 in 1943, the year that the Golden Globes launched. His songs have lived on for nearly a century and the Richard Linklater-directed Blue Moon opens at a time when…