The term “cosy crime” describes the reassuring, cardigan-swaddled whodunnits that currently dominate both page and screen, but it carries different connotations for Kelly Reichardt, director of the new heist movie The Mastermind, as it surely…
Blog
-

Virgin Australia makes history with Australia’s first Pets in Cabin flights
Virgin Australia has unleashed a new era of travel, becoming the first airline down under to launch a Pets in Cabin service.
The flights have kicked off with services between Melbourne, the Gold Coast, and the Sunshine Coast. Initially launching as a trial until 30 January 2026 before a planned rollout to all eligible domestic flights by late next year.
The service is already proving a hit for sunseekers, with more than half of all Pets in Cabin bookings to date made for the summer season. For many Australians, this means no more difficult goodbyes when the suncream and suitcases get rolled out.
This isn’t the airline’s first first either. Innovation runs in Virgin Australia’s DNA. From pioneering online check-in to introducing the Middle Seat Lottery (making life a little bit sweeter when you draw the short straw), and launching the country’s first Pride Flight – Virgin Australia has a track record of rewriting aviation norms.
To celebrate the inaugural furry flight (VA733 from Melbourne to the Gold Coast), guests were treated to 10,000 Velocity Frequent Flyer Points (enough to redeem a one-way domestic flight) and treated to a special in-flight announcement from none other than Richard Branson. As he put it: “This is history in the making, and I hope it’s making everyone smile”, before adding an obligatory, “woof, woof!”
Allowing pets to travel in the cabin speaks to Virgin Australia’s spirit of doing things differently to make flying just that little bit more wonderful. As the airline’s CEO Dave Emerson put it: “Pets are part of the family, and this new service is about making sure every member, human or furry, can enjoy the journey together. We’ve always believed in doing things differently, and this innovation continues that legacy.”
How it works, in case you’re curious…
Eligible small dogs and cats can travel for an introductory price of $149 per animal, per flight. Pets must travel in an approved soft-sided carrier that fits under the seat in front of their owner.
There will be a limit of four pets per flight, and guests travelling with pets will be seated in designated window seat rows to ensure comfort and safety for all passengers.
Virgin Australia’s advanced air filtration systems remove 99% of airborne particles, including pet dander, to ensure the cabin remains allergy-safe. Guests with allergies can also choose alternative seating, free of charge.
Bookings for Pets in Cabin can be made by calling the Virgin Australia Guest Contact Centre on 13 67 89.
Find more pet-friendly details right here.
Continue Reading
-

How companies are fostering the creation of human & AI agent teams
Many organizations are merging their HR and IT functions under unified leadership to create a more integrated, data-driven, and agile organization, but this convergence also presents complex challenges that must be addressed
Key takeaways:
-
-
-
AI is blurring the lines between HR and IT — The increasing integration of AI agents into workflows is prompting organizations to merge their HR and IT functions, with a significant percentage of IT leaders saying they expect this trend to continue.
-
Strategic actions for success — To effectively integrate HR and IT and leverage AI, organizations need strong, cross-functional leadership, a clear strategic direction for AI adoption, cultivation of AI literacy and adaptability, and a data-driven approach to cultural transformation.
-
Human adaptation is key — Unlocking organizational potential can only occur with thoughtful leadership that prioritizes human adaptation and intelligently orchestrates the integration of people and AI.
-
-
As AI continues to transforms the future of work, a growing number of organizations are breaking down traditional departmental silos by merging their HR and IT functions under unified leadership — and many expect this trend to continue in the future. Indeed, 64% of IT leaders surveyed say they believe HR and IT will merge within the next five years. This strategic convergence is a fundamental shift that is compelling organizational leaders to reimagine how work gets done while presenting other complex challenges.
The convergence of HR and IT functions
The rapid ascent of AI agents from current tools to a future state in which they are expected to be colleagues is blurring the lines between traditional technical and non-technical roles. As Skillsoft’s Chief People Officer Ciara Harrington recently stated: “There is no role that’s not a tech role.”
For many forward-thinking organizations, merging these departments offers a combination of benefits and paves the way for a more integrated, data-driven, and agile organization, that clearly offer some benefits including:
Holistic workforce architecture — Merging HR and IT enables leaders to design how work is done and better align human skills with hardware, software, and AI. For example, Moderna is reframing workforce planning around workflows by segmenting what technology should do and where humans add irreplaceable value, explains Tracey Franklin, the company’s chief people and digital technology officer.
Streamlined innovation and agility — When HR and IT co-own transformation, organizations can adapt faster to new tech and processes. In another example, Bunq, an online bank, organized its IT and people teams to sit within the same bigger team because they both are building systems that support the rest of the business.
Transformation brings about challenges
Navigating the risks of merging HR and IT is not without challenges, however, and organizations must carefully address several barriers to progress, which include:
Loss of specialist expertise — The most pressing concern involves diluting critical professional knowledge. “Merging the departments risks losing or diluting the specialist expertise organizations need to thrive,” warns David D’Souza from the Chartered Institute of Professional Development. Indeed, the skillsets of HR and IT professionals involve few areas of overlap.
Cognitive depletion — A risk that is just starting to get the attention it deserves is the danger of over-dependence on AI that can cause reduced cognitive capabilities. “If AI agents do everything with us, we lose skills,” says Skillsoft’s Harrington. Indeed, this long-term capability risk is multifaceted, resulting in core human skills atrophying and bench strength, adaptability, and ethical discernment weakening.
New roles, metrics, and leadership — Perhaps the most challenging areas are those that will determine who will manage the AI agents, how human-AI team performance will be evaluated, and what professional development looks like for hybrid human and AI agent teams. Answering these key questions remains an area of ongoing deliberation.
Likewise, traditional HR metrics don’t fit human-AI teams. Organizations must redefine performance, learning paths, and career progression for both humans and AI. In addition, organizations still need leaders who can navigate the human side of transformation as AI integration accelerates.
Key actions for companies
To unlock real returns on AI investments and from HR and IT integration, organizations need visionary, cross-functional leadership that sets clear strategy, aligns operations, and equips people to work differently. Must-do actions include:
Developing strategic direction — Research in the recent Thomson Reuters Future of Professionals Report 2025 identifies four interconnected layers for AI success, which include a clear, visible plan for AI adoption (strategy), committed leaders who model the right behaviors (leadership), adjusted workflows and roles to leverage AI (operations), and ways to empower people to learn and set personal AI goals (individuals).
While organizations that align all four layers unlock the greatest value from AI, the first layer, strategy, is the single strongest predictor of AI return on investment, according to the report. And the same is true for successful HR and IT integration. It requires leaders who can bridge both worlds without necessarily being technical experts, while also setting direction and providing vision, allocating capital effectively, removing obstacles, fostering culture, and engaging employees.
Cultivating AI literacy and adaptability — Organizations also must develop comprehensive AI training with responsible AI, including clear usage policies. This includes preparing employees to incentivize experimentation around recreating their own workflows to allow AI to execute repetitive tasks while team members can then focus on more complex problem-solving.
Data-driven cultural transformation — Success requires using data strategically to transform the culture to shift to collaborative human/AI ecosystems. Some companies are using data and building accountability mechanisms to ensure leaders are culture promoters and data stewards. Without this data-centric approach to cultural change, organizations likely will fail to realize the full potential of integrated HR and IT functions.
Yet, no matter what functions are merged, organizational potential is unlocked by thoughtful leadership that centers human adaptation and intentionally orchestrates how people and AI integrate to do the work. Now is the moment for companies to define a clear roadmap, invest in capability-building, and pilot human/AI teams with measurable guardrails to help organizations learn fast, acclimate to new work realities, and scale what works.
You can find out more about how organizations are addressing issues of talent development and management here
Continue Reading
-
-

Cancer-Related Cause of Death and End-of-Life Management in Patients With ALK-Rearranged NSCLC
Patients with ALK-positive (ALK+) non-small cell lung cancer (NSCLC) “frequently die of cancer-related causes, during hospitalization, and under active treatment,” underscoring the need for tailored palliative and end-of-life care approaches…
Continue Reading
-

‘Emily in Paris’ Season 5 Teaser Trailer: Marcello Romance in Rome
Lily Collins‘ Emily Cooper is feeling the love in Rome in the latest teaser trailer for Emily in Paris‘ fifth season, all 10 episodes of which start streaming on Netflix Dec. 18.
The preview highlights her romance with Marcello…
Continue Reading
-

Milano Cortina 2026 Olympic Flame-Lighting and Handover Ceremonies – Information for the Media
The Olympic flame will first be carried through Greece before arriving in Athens for the Handover Ceremony with a delegation from the Milano Cortina 2026 Organising Committee at the Panathenaic Stadium on Thursday 4 December 2025.
Following the…
Continue Reading
-

Astronomers discover skyscraper-size asteroid hidden in sun’s glare — and it’s moving at a near-record pace
Astronomers have discovered a 2,300-foot-wide (700 meters) asteroid hidden in the sun’s glare, and it’s whizzing through our solar system at a near record-breaking pace.
The skyscraper-size asteroid, named 2025 SC79, loops around the sun once…
Continue Reading
-

Neutrino Experiments in U.S. and Japan Join Forces
Caltech researchers co-lead new study refining what we know about the ghostly particles
Very early on in our universe, when it was a seething hot cauldron of energy, particles made of matter and antimatter bubbled into existence in equal…
Continue Reading
-

Armata Pharmaceuticals Highlights Positive Results from Phase 2a diSArm Study of its Staphylococcus aureus Bacteriophage Cocktail, AP-SA02, in Late-Breaking Oral Presentation at IDWeek 2025™
LOS ANGELES, Oct. 22, 2025 /PRNewswire/ — Armata Pharmaceuticals, Inc. (NYSE American: ARMP) (“Armata” or the “Company”), a clinical-stage biotechnology company focused on the development of high-purity, pathogen-specific bacteriophage therapeutics for the treatment of antibiotic-resistant and difficult-to-treat bacterial infections, today highlighted positive results from its recently completed Phase 2a diSArm study of AP-SA02 as a potential treatment for complicated Staphylococcus aureus (“S. aureus“) bacteremia (“SAB”) in a late-breaking oral presentation at IDWeek 2025™.
The abstract, titled, “A Phase 2a Randomized, Double-Blind, Controlled Trial of the Efficacy and Safety of an Intravenous (IV) Bacteriophage Cocktail (AP-SA02) vs. Placebo in Combination with Best Available Antibiotic Therapy (BAT) in Patients with Complicated Staphylococcus aureus Bacteremia,” was accepted as a highly coveted late-breaking abstract for oral presentation, and was presented by Dr. Loren G. Miller, M.D., M.P.H., Professor of Medicine, David Geffen School of Medicine at UCLA, Chief, Division of Infectious Diseases at Harbor-UCLA Medical Center and the Lundquist Institute.
“The results of the diSArm study confirm, for the first time in a randomized clinical trial, the efficacy of intravenous phage therapy for S. aureus bacteremia, and we are very pleased to highlight these compelling data in an oral presentation at IDWeek,” stated Dr. Miller. “The results of this rigorously designed study provide strong rationale for advancement into a Phase 3 superiority study that, if successful, would support its use in clinical practice for Staphylococcus aureus bacteremia. High-purity, phage-based therapeutics like AP-SA02 have the potential to become the new standard of care for this common, extremely severe, and often deadly infection.”
“The positive results from the diSArm study represent another significant achievement for Armata as we aim to advance AP-SA02 into a pivotal trial,” stated Dr. Deborah Birx, Chief Executive Officer of Armata. “I would like to thank Dr. Miller and the other investigators who contributed to the efficient execution of the diSArm study, and I look forward to working with many of them on a proposed pivotal study next year. I would also like to thank Dr. Vance Fowler who served as the chair of the independent blinded adjudication committee that independently confirmed safety and efficacy findings throughout the Phase 2 trial. Finally, I would like to express my gratitude to the patients who participated in this important study, and acknowledge our partners at the U.S. Department of Defense, and our significant shareholder, Innoviva, who have each provided critical support to make this early breakthrough possible.”
Data highlights:
The Phase 2a study enrolled and dosed 42 patients, with 29 randomized to AP-SA02 in addition to BAT and 13 to placebo (BAT alone). Methicillin-resistant S. aureus (“MRSA”) was the causative pathogen in ~38% of both the AP-SA02 and placebo groups.
Clinical response was assessed in the intent-to-treat (ITT) population at Test of Cure (“TOC”) on day 12, one week post-BAT, and End of Study (“EOS”) four weeks after BAT completion. Safety analysis also included data from the Phase 1b portion of the trial (n=8).
Day 12 clinical response rates were higher in the AP-SA02 group — 88% (21/24) versus 58% (7/12) in the placebo group as assessed by blinded site investigators (“PI”) (p = 0.047), and 83% (20/24) in the AP-SA02 group versus 58% (7/12) in the placebo group as assessed by the blinded Adjudication Committee (“AC”).
Non-response/relapse rates were evaluated at the two later timepoints — one week post-BAT and EOS. No patients in the AP-SA02 group experienced non-response or relapse (0%) by either PI or AC assessment. In contrast, the placebo group showed 25% non-response/relapse at both timepoints reported by the PI (p = 0.017) and 22% non-response/relapse at one week post-BAT (p = 0.025) and 25% at EOS (p = 0.02) by the AC.
Patients treated with AP-SA02 showed trends toward rapid normalization of C-reactive protein, shorter time to negative blood culture, quicker time to resolution of signs and symptoms at the infection site, shorter intensive care unit and hospital utilization.
AP-SA02 was well-tolerated with no serious adverse events related to the study drug. Treatment-emergent adverse events occurred in 6% (2/35) and 0% (0/15) in the AP-SA02 and placebo groups, respectively: one patient with transient liver enzyme elevation and one patient with hypersensitivity that resolved with discontinuation of vancomycin.
New findings demonstrate that the defined and reproducible genomic variants present in AP-SA02 Drug Product may provide an immediate advantage, enabling rapid, strain-specific response to each patient’s S. aureus isolate. These characterized variants can expand from as little as 2% to dominance when infecting certain patient isolates in vitro, highlighting that these variants are favored for their enhanced ability to infect those strains and the importance of integrating this diversity into Armata’s phage cocktail from the outset. This inherent flexibility may be central to achieving optimal therapeutic efficacy.
Conclusions:
- AP-SA02, combined with BAT, had a higher and earlier cure rate compared to placebo in patients with complicated SAB at day 12 as assessed by both blinded site investigators and independent adjudicators.
- No patients who received AP-SA02 demonstrated non-response or relapse at one week post-BAT or at EOS, as assessed by both blinded site investigators and the independent adjudication committee, compared with approximately 25% in the placebo group.
- AP-SA02 appears safe with clinical efficacy against both MRSA and methicillin-sensitive S. aureus (“MSSA”) and trends toward earlier resolution and shorter hospitalization, with no evidence of relapse four weeks post-therapy.
- Defined phage variants in AP-SA02 Drug Product ensure an intrinsic adaptive mechanism — a flexibility that may be key to achieving effective phage therapy from patient to patient.
- These results strongly support advancement into a pivotal Phase 3 trial that Armata plans to initiate in 2026, subject to review and feedback from the U.S. Food and Drug Administration (the “FDA”). The Company is engaged with the FDA regarding a potential superiority trial design.
About IDWeek 2025™
IDWeek 2025™ is a joint annual meeting of the Infectious Diseases Society of America (IDSA), the Society for Healthcare Epidemiology of America (SHEA), the HIV Medicine Association (HIVMA), the Pediatric Infectious Diseases Society (PIDS) and the Society of Infectious Diseases Pharmacists (SIDP). With the theme “Advancing Science, Improving Care,” IDWeek features the latest science and bench-to-bedside approaches in prevention, diagnosis, treatment and epidemiology of infectious diseases, including HIV, across the lifespan. IDWeek 2025™ takes place October 19-22 in Atlanta, GA. For more information, visit www.idweek.org.
About AP-SA02 and diSArm Study
Armata is developing AP-SA02, a fixed multi-phage phage cocktail, for the treatment of complicated bacteremia caused by Staphylococcus aureus (S. aureus), including methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) strains.The diSArm study (NCT05184764) was a Phase 1b/2a, multicenter, randomized, double-blind, placebo-controlled, multiple ascending dose escalation study of the safety, tolerability, and efficacy of intravenous AP-SA02 in addition to best available antibiotic therapy (BAT) compared to BAT alone (placebo) for the treatment of adults with complicated S. aureus bacteremia. The results from the diSArm study are an important step forward in Armata’s effort to confirm the potent antimicrobial activity of phage therapy and the completion of the study represents a significant milestone in the development of AP-SA02, moving Armata one step closer to introducing an effective new treatment option to patients suffering from complicated S. aureus bacteremia.
The Phase 1b/2a clinical development of AP-SA02 was partially supported by a $26.2 million Department of Defense (DoD) award, received through the Medical Technology Enterprise Consortium (MTEC) and managed by the Naval Medical Research Command (NMRC) – Naval Advanced Medical Development (NAMD) with funding from the Defense Health Agency and Joint Warfighter Medical Research Program.
About Armata Pharmaceuticals, Inc.
Armata is a clinical-stage biotechnology company focused on the development of high-purity pathogen-specific bacteriophage therapeutics for the treatment of antibiotic-resistant and difficult-to-treat bacterial infections using its proprietary bacteriophage-based technology. Armata is developing and advancing a broad pipeline of natural and synthetic phage candidates, including clinical candidates for Pseudomonas aeruginosa, Staphylococcus aureus, and other important pathogens. Armata is committed to advancing phage therapy with drug development expertise that spans bench to clinic including in-house phage-specific current Good Manufacturing Practices (“cGMP”) manufacturing to support full commercialization.Forward Looking Statements
This communication contains “forward-looking” statements as defined by the Private Securities Litigation Reform Act of 1995. These statements relate to future events, results or to Armata’s future financial performance and involve known and unknown risks, uncertainties and other factors which may cause Armata’s actual results, performance or events to be materially different from any future results, performance or events expressed or implied by the forward-looking statements. In some cases, you can identify these statements by terms such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” or the negative of those terms, and similar expressions. These forward-looking statements reflect management’s beliefs and views with respect to future events and are based on estimates and assumptions as of the date of this communication and are subject to risks and uncertainties including risks related to Armata’s development of bacteriophage-based therapies; ability to staff and maintain its production facilities under fully compliant cGMP; ability to meet anticipated milestones in the development and testing of the relevant product; ability to be a leader in the development of phage-based therapeutics; ability to achieve its vision, including improvements through engineering and success of clinical trials; ability to successfully complete preclinical and clinical development of, and obtain regulatory approval of its product candidates and commercialize any approved products on its expected timeframes or at all; and Armata’s estimates regarding anticipated operating losses, capital requirements and needs for additional funds. Additional risks and uncertainties relating to Armata and its business can be found under the caption “Risk Factors” and elsewhere in Armata’s filings and reports with the U.S. Securities and Exchange Commission (the “SEC”), including in Armata’s Annual Report on Form 10-K, filed with the SEC on March 21, 2025, and in its subsequent filings with the SEC.Armata expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in Armata’s expectations with regard thereto or any change in events, conditions or circumstances on which any such statements are based.
Media Contacts:
At Armata:
Pierre Kyme
[email protected]
310-665-2928Investor Relations:
Joyce Allaire
LifeSci Advisors, LLC
[email protected]
212-915-2569SOURCE Armata Pharmaceuticals, Inc.
Continue Reading
-
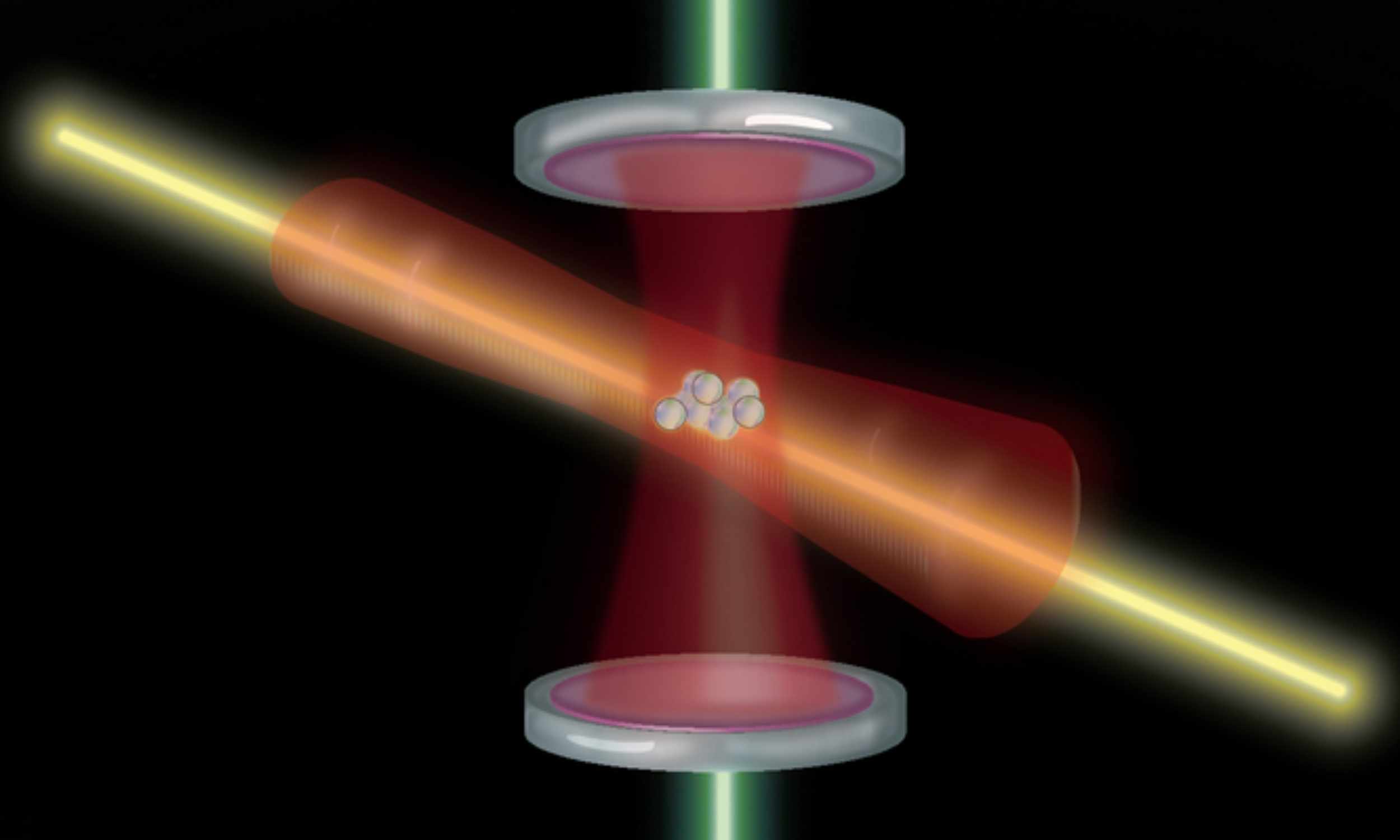
Atomic clock doubles its accuracy by reducing quantum noise
Optical atomic clocks just took a clean step forward. An MIT team reports a method that cuts quantum noise and lets a clock resolve twice as many ticks as before.
The work centers on ytterbium atoms and a new way to steady a laser, and careful…
Continue Reading
