Redmagic 11 pro smartphone cools down with flowing water
Redmagic unveils the 11 Pro smartphone, which uses visible flowing water as its liquid cooling technology to keep the device’s temperature low. To be released starting November 3rd,…

Redmagic unveils the 11 Pro smartphone, which uses visible flowing water as its liquid cooling technology to keep the device’s temperature low. To be released starting November 3rd,…

American racing icon Wayne Taylor has taken the start in the 24 Hours of Le Mans a remarkable 12 times. This year, he competed as team manager of Wayne Taylor Racing in the Hypercar category with a Cadillac V-Series.R after receiving an…

All products and services featured are independently chosen by editors. However, Billboard may receive a commission on orders placed through its retail links, and the retailer may receive certain auditable data for accounting purposes.
Have…

Parliament is under mounting pressure to examine what the royal family knew about Prince Andrew’s links to Jeffrey Epstein and introduce a mechanism to strip him of his titles.
There were calls on Sunday night for Andrew to face a police…

Even in the fast-moving world of AI, patience is still a virtue, according to Andrej Karpathy.
The OpenAI cofounder, and de facto leader of the vibe-coding boom, appeared on the Dwarkesh Podcast last week to talk about how far we are from developing functional AI agents.
TL;DR — he’s not that impressed.
“They just don’t work. They don’t have enough intelligence, they’re not multimodal enough, they can’t do computer use and all this stuff,” he said. “They don’t have continual learning. You can’t just tell them something and they’ll remember it. They’re cognitively lacking and it’s just not working.”
“It will take about a decade to work through all of those issues,” he added.
Agents are among the most talked-about innovations in AI, with many investors dubbing 2025 “the year of the agent.” While definitions vary, agents are virtual assistants capable of completing tasks autonomously — breaking down problems, outlining plans, and taking action without user prompts.
Karpathy is a famously fast talker. So he wrote a follow-up post on X for listeners who couldn’t quite parse everything he said. On the topic of agents, he reiterated his earlier frustrations.
“My critique of the industry is more in overshooting the tooling w.r.t. present capability,” he wrote. “The industry lives in a future where fully autonomous entities collaborate in parallel to write all the code and humans are useless.”
He doesn’t want to live there.
In Karpathy’s ideal future, humans and AI collaborate to code and execute tasks.
“I want it to pull the API docs and show me that it used things correctly. I want it to make fewer assumptions and ask/collaborate with me when not sure about something. I want to learn along the way and become better as a programmer, not just get served mountains of code that I’m told works,” he wrote.
The con of building the kind of agents that render humans useless, he said, is that humans are then useless, and AI “slop,” the low-quality content generated by AI, becomes ubiquitous.
Karpathy isn’t the only one to raise concerns about the functionality of AI agents.
In a post on LinkedIn last year, ScaleAI growth lead Quintin Au talked about how the errors agents make are compounded with every additional task they take on.
“Currently, every time an AI performs an action, there’s roughly a 20% chance of error (this is how LLMs work, we can’t expect 100% accuracy),” he wrote in a post on LinkedIn. “If an agent needs to complete 5 actions to finish a task, there’s only a 32% chance it gets every step right.”
While skeptical of the current state of AI agents, Karpathy said he isn’t an AI skeptic.
“My AI timelines are about 5-10X pessimistic w.r.t. what you’ll find in your neighborhood SF AI house party or on your twitter timeline, but still quite optimistic w.r.t. a rising tide of AI deniers and skeptics,” he said.

“God Save the King” has never been the loveliest or most melodic of national anthems, and its somewhat chiding, aggressive tenor is brought to the fore early in “The Choral.” Upon delivery of some good news from the front in the grim…
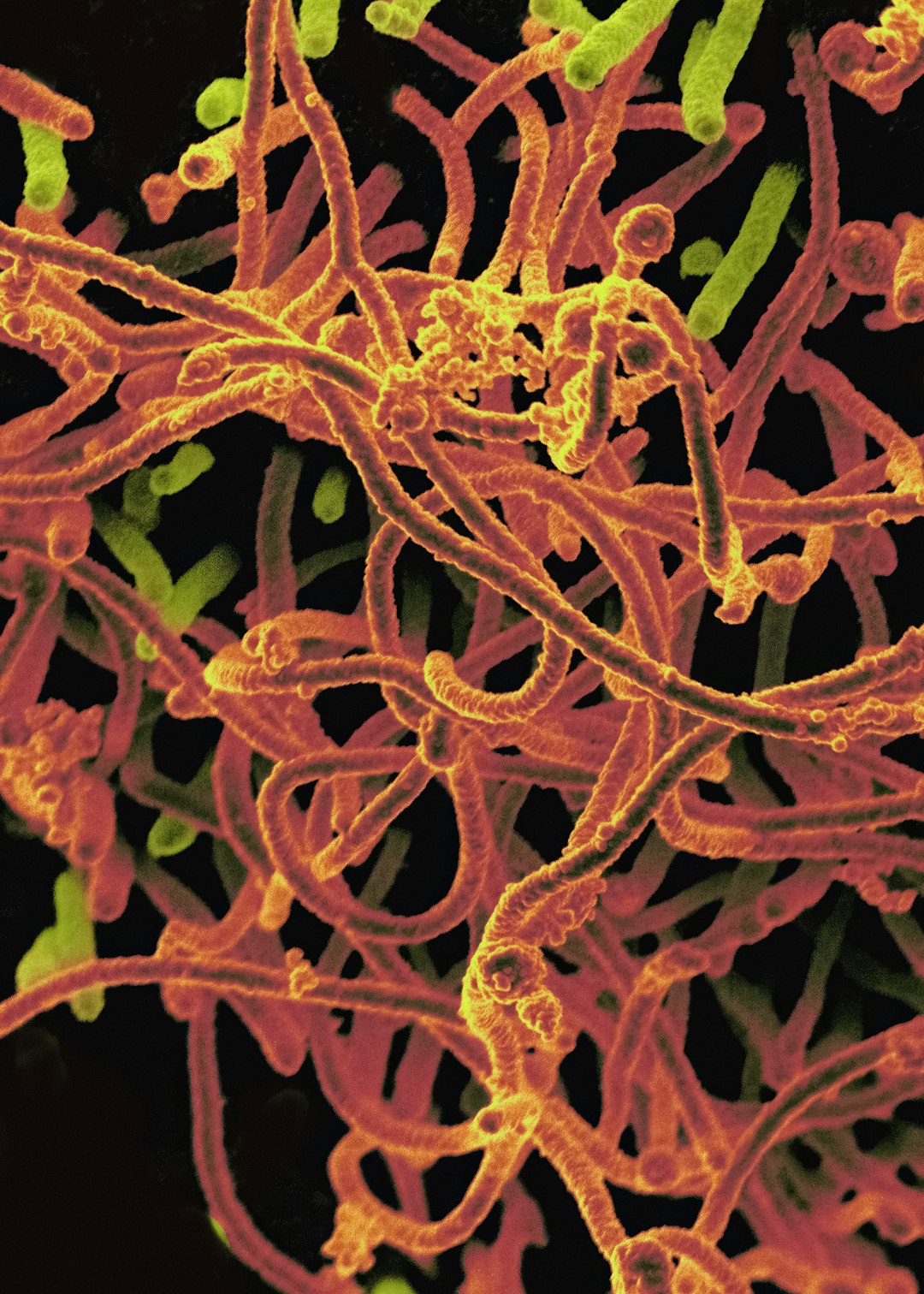
The World Health Organization (WHO) reported today that the last Ebola patient in the Democratic Republic of the Congo was discharged today. No new cases have been reported since 25 September.
This starts the 42-day countdown to declaring the…
Sirexatamab (DKN-01) in combination with bevacizumab (Avastin) and standard-of-care (SOC) chemotherapy demonstrated a manageable safety profile and was well tolerated vs bevacizumab plus SOC chemotherapy in patients with metastatic colorectal cancer (mCRC), according to findings from the phase 2 DeFianCe trial (NCT05480306) presented at the
In the intent-to-treat population, patients who received the DKK1 inhibiting IgG4 antibody sirexatamab demonstrated an overall response rate (ORR) of 35.1% (95% CI, 25.5%-45.6%) vs 26.6% (95% CI, 18.0%-36.7%) for the control arm (P = .10). Median PFS was 9.2 months in the experimental arm vs 8.3 months in the control arm (P = .17).
“Neither of these 2 outcomes reached statistical significance,” lead author Zev A. Wainberg, MD, said during the presentation of data. “However, looking at the DKK1-high population, defined as the upper median, the improvement started to bear fruit,” Wainberg, professor of medicine at UCLA and codirector, UCLA GI program, at UCLA Health in California continued.
There were 50 patients with higher DKK1 in the sirexatamab arm and 38 in the control arm. The ORR in the sirexatamab arm was 38.0% (95% CI, 24.7%-52.8%) vs 23.7% (95% CI, 11.4%-40.2%) in the control arm (P = .0706). Median PFS in the experimental arm was 9.03 months vs 7.06 months (HR, 0.61; 95% CI, 0.37-1.0; P = .0255). Median OS was not reached in the experimental arm vs 14.39 months in the control arm (HR, 0.42; 95% CI, 0.19-0.91; P = .0118).
After an initial safety run-in period (n = 33), a total of 188 patients with mCRC were randomly assigned 1:1 to receive either sirexatamab plus either leucovorin, 5-fluorouracil (5-FU), and irinotecan (FOLFIRI) or leucovorin, 5-FU, and oxaliplatin (FOLFOX) and bevacizumab (n = 94) vs FOLFIRI or FOLFOX and bevacizumab (n = 94).
Patients were eligible if they had received 1 prior 5-FU–based therapy, had microsatellite stable (MSS) CRC, and did not have a BRAF V600 mutation. The stratification factors were tumor sidedness (left vs right) and prior antiangiogenesis therapy (yes vs no).
The primary end point was investigator-assessed PFS and the secondary end points were safety, ORR, and OS. Key exploratory end points were PFS, ORR, and the OS of the DKK1-high subgroup.
The study was well-balanced for gender and region. The majority of patients were male across the arms (experimental, 68%; control, 55%). In the experimental arm, the majority of patients were from South Korea (53%) compared with 48% in the control arm. Forty-four percent of patients were from the United States in the experimental arm and 43% in the control arm. The majority of patients had left-sided tumors (both 75%).
“This was a predominantly liver-metastatic patient population, with nearly 75% in both arms having liver metastatic disease,” Wainberg said. Further, 47% of patients in the experimental arm had RAS-mutated disease compared with 57% in the control arm. Looking at prior systemic therapy, 48% of patients in the experimental arm had received antiangiogenesis therapy compared with 51% in the control arm.
The overall rate of treatment-emergent adverse events (TEAEs) for sirexatamab was similar to the chemotherapy arm, suggesting that the addition of the agent does not adversely impact the safety profile of the combined agents.
In the experimental arm, 15% of patients discontinued therapy compared with 19% in the control arm. In the patients with DKK1-high status, 4% discontinued in the treatment arm vs not applicable in the control arm. Dose reductions affected 37% of patients in the experimental arm compared with 43% in the control arm. Dose interruptions were similar, with 73% in the experimental arm and 71% in the control arm.
“These data support continued development of sirexatamab in DKK1-high previously treated patients with mCRC,” Wainberg concluded.
Disclosures: Dr Wainberg disclosed that he has provided consulting services to Alligator Therapeutics, Amgen, AstraZeneca, Arcus, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo Company, Eli Lilly and Company, EMD Serono, Roche AG, Genentech, Ipsen, Johnson & Johnson, Merus NV, Merck, Novartis, Novocure, Pfizer, Servier, and Verastem.
Wainberg ZA, Han S-W, Kim JG, et al. DeFianCe Trial: A randomized phase 2 trial of sirexatamab (DKN-01) plus bevacizumab and chemotherapy versus bevacizumab and chemotherapy as second-line therapy in advanced microsatellite stable (MSS) colorectal cancer (CRC). Presented at: 2025 European Society for Medical Oncology Congress; October 17-21, 2025; Berlin, Germany. Abstract LBA34.
(Web Desk) – New evidence has found that male brains really may shrink faster than female brains with age.
Among 4,726 participants with healthy cognition, brain scans have revealed “modest yet systematic sex…