…
Blog
-
Sinner, Fritz, Shelton headline Vienna & Basel fields in potentially crucial week in Live Race – ATP Tour
- Sinner, Fritz, Shelton headline Vienna & Basel fields in potentially crucial week in Live Race ATP Tour
- Sinner in line for Bublik rematch in Vienna, Musetti & Tsitsipas in R1 showdown ATP Tour
- Sinner vs. Altmaier Prediction at the Erste Bank Open…
Continue Reading
-
OSCE Supports Dialogue and Unity at the First Fergana Peace Forum
First Fergana Peace Forum was organized from 15 to 16 October 2025 at Fergana university. More than 300 participants from Central Asia, the CIS, Europe, Asia and the America gathered to discuss under the title “Uniting efforts for peace…
Continue Reading
-

KP health department launches probe into Marian Dengue deaths
– Advertisement –
PESHAWAR, Oct 19 (APP):The Khyber Pakhtunkhwa health department has ordered a comprehensive investigation into the alleged dengue-related deaths of two patients at a hospital in Mardan.
The directive, issued by Secretary of…
Continue Reading
-

Governor KP, Mohsin Naqvi confer on peace and stability in the province
Governor Khyber Pakhtunkhwa Faisal Karim Kundi met with Federal Interior Minister Mohsin Naqvi at his residence in Lahore on Sunday to discuss the overall law and order situation in the province and recent incidents of terrorism.
…
Continue Reading
-
Final Analysis of COMPASSION-15 Presented at ESMO 2025
HONG KONG, Oct. 19, 2025 /PRNewswire/ — On October 19, 2025, Akeso (9926.HK) announced the final analysis results from the COMPASSION-15/AK104-302 study at the 2025 European Society of Medical Oncology Congress (ESMO 2025) . COMPASSION-15 is a Phase III clinical trial evaluating cadonilimab, Akeso’s first-in-class PD-1/CTLA-4 bispecific antibody, in combination with oxaliplatin and capecitabine as first-line treatment for unresectable, locally advanced, recurrent, or metastatic gastric or gastroesophageal junction (G/GEJ) adenocarcinoma. Professor Shen Lin, principal investigator from Peking University Cancer Hospital, presented the findings in an oral session at ESMO 2025.
In this final analysis presented at ESMO 2025 (median follow-up: 33.9 months), the cadonilimab regimen demonstrated enhanced long-term survival benefits in first-line advanced G/GEJ adenocarcinoma. With the extended follow-up period and more mature data, the cadonilimab treatment regimen showed a further reduction in the risk of death compared to the control group. This consistent benefit was observed across all PD-L1 expression subgroups.
The interim analysis of COMPASSION-15, with a median follow-up of 18.7 months, was previously published in Nature Medicine in January 2025. The data presented at ESMO 2025 were analyzed using the same statistical methodology.
COMPASSION-15 2025 ESMO Data
In the intent-to-treat (ITT) population:
- With long-term follow-up, the cadonilimab regimen demonstrated a significant 39% reduction in the risk of death (OS HR 0.61) versus the control group, showing further improvement over the data from the median 18.7-month follow-up (OS HR 0.66).
- With extended follow-up, in the PD-L1 CPS ≥5 population, the cadonilimab regimen demonstrated a significant 51% reduction in the risk of death (OS HR 0.49; p < 0.001) compared to the control group, showing further improvement over the data from the median 18.7-month follow-up (OS HR 0.58).
- With long-term follow-up, in the PD-L1 CPS <5 population, the cadonilimab regimen showed a significant 24% reduction in the risk of death (OS HR 0.76; 95% CI: 0.59-0.99; p = 0.019) versus the control group, with a strengthening trend of benefit compared to the data from the median 18.7-month follow-up (OS HR 0.75; 95% CI: 0.56-1.00).
- Following extended the follow-up period, the cadonilimab combination regimen maintained a favorable safety profile, with no new safety signals emerging.
In the COMPASSION-15 study, patients with PD-L1 CPS <5 (low expression) and CPS <1 (negative expression) are 49.8% and 23%, respectively, of the ITT population. This represents a higher proportion of PD-L1 low and negative patient population in COMPASSION-15 compared to previous Phase III trials of other immune checkpoint inhibitors used in the treatment of first-line gastric cancer. Previous studies have shown limited responses to PD-1/L1 inhibitors in PD-L1 low-expression or negative patients.
Cadonilimab was approved by the NMPA in September 2024 for the first-line treatment for advanced gastric cancer, offering a new and effective immunotherapy option. Cadonilimab has been included in the 2025 CSCO Gastric Cancer Guidelines as the only Category I recommendation (Level 1A evidence) for first-line immunotherapy, regardless of PD-L1 expression, and is currently widely used in clinical practice.
Forward-Looking Statement of Akeso, Inc.
This announcement by Akeso, Inc. (9926.HK, “Akeso”) contains “forward-looking statements”. These statements reflect the current beliefs and expectations of Akeso’s management and are subject to significant risks and uncertainties. These statements are not intended to form the basis of any investment decision or any decision to purchase securities of Akeso. There can be no assurance that the drug candidate(s) indicated in this announcement or Akeso’s other pipeline candidates will obtain the required regulatory approvals or achieve commercial success. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and health care legislation in P.R.China, the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; Akeso’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the Akeso’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
Akeso does not undertake any obligation to publicly revise these forward-looking statements to reflect events or circumstances after the date hereof, except as required by law.
About Akeso
Akeso (HKEX: 9926.HK) is a leading biopharmaceutical company committed to the research, development, manufacturing and commercialization of the world’s first or best-in-class innovative biological medicines. Founded in 2012, the company has created a unique integrated R&D innovation system with the comprehensive end-to-end drug development platform (ACE Platform) and bi-specific antibody drug development technology (Tetrabody) as the core, a GMP-compliant manufacturing system and a commercialization system with an advanced operation mode, and has gradually developed into a globally competitive biopharmaceutical company focused on innovative solutions. With fully integrated multi-functional platform, Akeso is internally working on a robust pipeline of over 50 innovative assets in the fields of cancer, autoimmune disease, inflammation, metabolic disease and other major diseases. Among them, 24 candidates have entered clinical trials (including 15 bispecific/multispecific antibodies and bispecific ADCs. Additionally, 7 new drugs are commercially available. Through efficient and breakthrough R&D innovation, Akeso always integrates superior global resources, develops the first-in-class and best-in-class new drugs, provides affordable therapeutic antibodies for patients worldwide, and continuously creates more commercial and social values to become a global leading biopharmaceutical enterprise.For more information, please visit https://www.akesobio.com/en/about-us/corporate-profile/ and follow us on Linkedin.
SOURCE Akeso, Inc.

Continue Reading
-
Interior minister briefs PM Shehbaz on law and order situation – Dawn
- Interior minister briefs PM Shehbaz on law and order situation Dawn
- TLP leadership to face music: Naqvi The Express Tribune
- No one will be allowed to spread unrest, warn federal ministers The Nation (Pakistan )
- Naqvi meets Owais Noorani Daily…
Continue Reading
-

Vierge and Lecuona round out the 2025 season…
After a final warm-up, the factory team turned its attentions to the Superpole race. Having qualified eighth, Vierge was straight up into fifth with his CBR1000RR-R. Passing Lowes for fourth on lap two, the home rider was lapping faster than…
Continue Reading
-

Bangladesh garment exporters fear $1bn losses after huge airport fire | Business and Economy News
The fire gutted import cargo terminals areas at Dhaka airport, destroying an estimated $1bn of ‘urgent air shipments’.
Published On 19 Oct 2025
A fire that decimated a cargo complex in…
Continue Reading
-
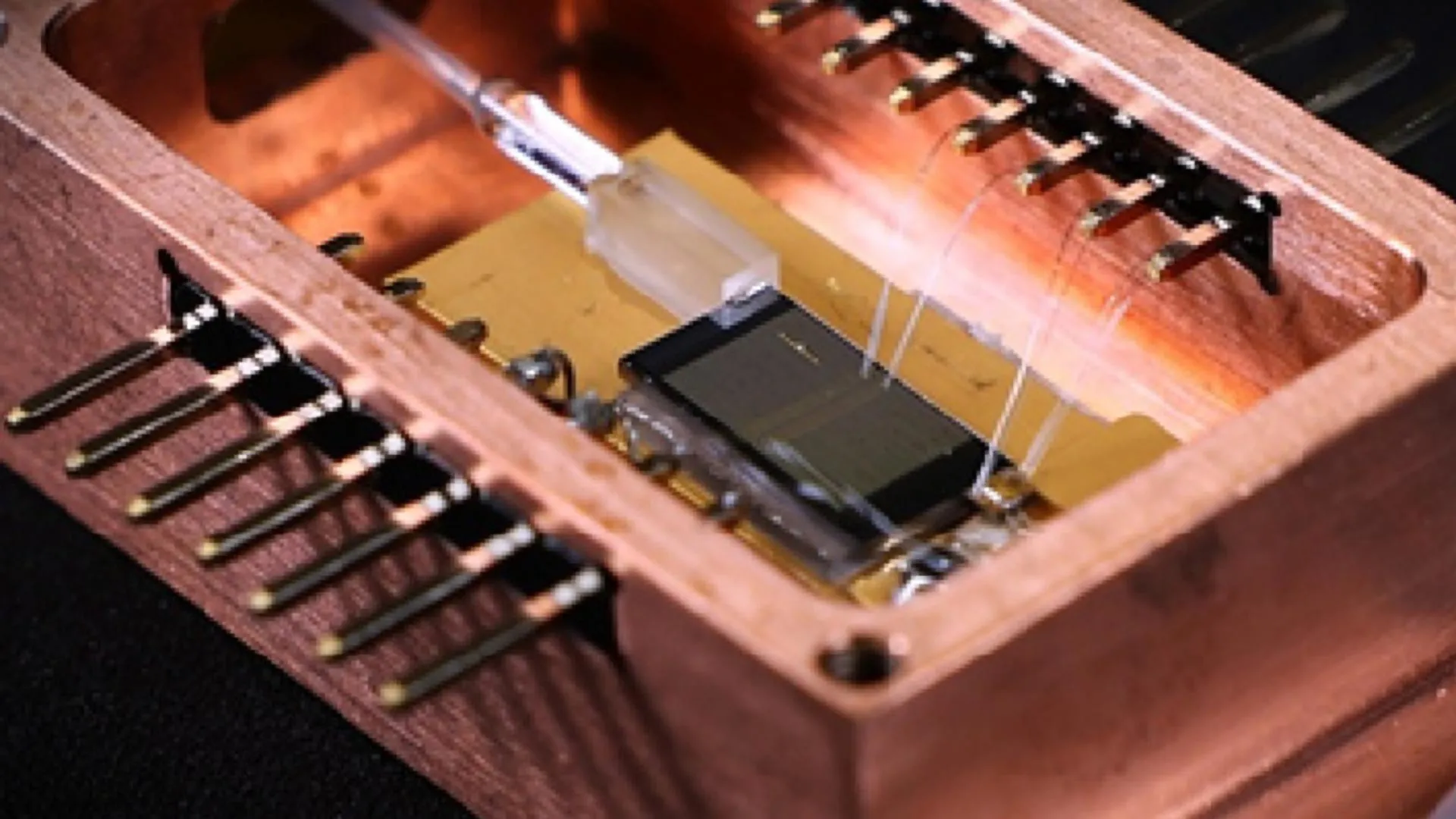
This tiny laser could transform how we see and sense the world
Laser technology plays a vital role in modern life, supporting everything from precise scientific measurements to advanced communication systems. It underpins technologies such as self-driving vehicles, high-speed fiber optic networks, and even…
Continue Reading
