A total of 222 Indian athletes are representing their country at the Asian Youth Games 2025, scheduled in Manama, Bahrain, from October 22 to 31.
The third edition of the continental event – returning after a 12-year gap since Nanjing 2013 in the…

A total of 222 Indian athletes are representing their country at the Asian Youth Games 2025, scheduled in Manama, Bahrain, from October 22 to 31.
The third edition of the continental event – returning after a 12-year gap since Nanjing 2013 in the…

The American racer is currently among the stars in F1 Academy, where she has quickly become one of the series’ stars, taking two race wins, four pole positions and ten podium finishes to-date. She is…

One of the most famous clusters in the sky, the Pleiades leads the figure of Taurus the Bull above the horizon on autumn evenings.

At the ESMO Congress 2025 in Berlin, Dr. Martina Carullo (Pisa, Italy) presented groundbreaking findings from the translational program of AtezoTRIBE and AVETRIC, two clinical trials exploring immunotherapy in proficient mismatch repair (pMMR) metastatic colorectal cancer (mCRC). Using the Lunit SCOPE IO artificial intelligence (AI) platform, the investigators developed and validated a digital biomarker capable of predicting which pMMR tumors benefit from immune checkpoint inhibition—an area long considered resistant to immunotherapy.
Immune checkpoint inhibitors (ICIs) have transformed treatment outcomes for patients with deficient mismatch repair (dMMR/MSI-H) mCRC, but have shown minimal activity in pMMR tumors, which constitute the vast majority of metastatic cases. Identifying predictive markers within this refractory group remains one of the central challenges in gastrointestinal oncology.
Recent advances in AI-driven pathology have enabled quantitative analysis of the tumor microenvironment on digitized hematoxylin and eosin (H&E) slides. By mapping immune, stromal, and tumor cell populations, AI models can capture subtle biological patterns invisible to traditional pathology. The present study used this approach to generate an AI-derived biomarker that could distinguish responders from non-responders to ICI-based regimens in pMMR mCRC.
The analysis incorporated pre-treatment tumor slides from patients enrolled in AtezoTRIBE (NCT03721653) and AVETRIC (NCT04513951). In AtezoTRIBE, patients received FOLFOXIRI/bevacizumab with or without atezolizumab, while in AVETRIC, the regimen was FOLFOXIRI/cetuximab/avelumab.
Using the Lunit SCOPE IO platform, the research team quantified the density of lymphocytes, fibroblasts, macrophages, endothelial, mitotic, and tumor cells within both cancer areas and surrounding stroma. A multivariate Cox regression model was trained on the atezolizumab-treated arm of AtezoTRIBE to identify cellular features most predictive of progression-free survival (PFS). A cut-off optimized for PFS was then applied to classify tumors as biomarker-high or biomarker-low, with AVETRIC serving as an external validation set.
The AI-based analysis was conducted on whole-slide images from 161 patients. The resulting biomarker integrated densities of tumor and mitotic cells in the cancer area, lymphocytes in the tumor core, and fibroblasts, macrophages, and endothelial cells in the stroma. Of the evaluated patients, 113 (70%) were classified as biomarker-high, a group characterized by older age (p = 0.030) and a higher frequency of liver metastases (p = 0.023).
In the atezolizumab arm, biomarker-high patients achieved significantly superior outcomes compared with biomarker-low ones, with PFS p = 0.036 and overall survival (OS) p = 0.024. No such association was observed in the control arm (PFS p = 0.564; OS p = 0.186).
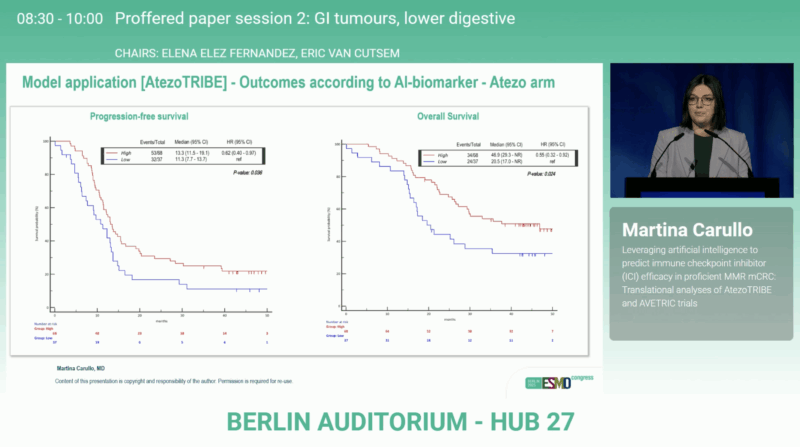
A formal treatment–biomarker interaction analysis showed a stronger benefit from atezolizumab among biomarker-high patients (HR for PFS 0.69; 95% CI 0.45–1.04 and HR for OS 0.54; 95% CI 0.33–0.88), while biomarker-low tumors did not derive advantage (HR for PFS 1.34; 95% CI 0.66–2.72 and HR for OS 1.70; 95% CI 0.69–4.20).
The model was independently tested on 48 patients from the AVETRIC trial. Thirty-six (75%) were classified as biomarker-high and displayed numerically improved outcomes compared with biomarker-low cases, with PFS p = 0.043and OS p = 0.053. The validation confirmed the reproducibility of the AI-generated signature across different ICI-based regimens and patient populations.
This dual-trial analysis demonstrates that an AI-defined histologic signature reflecting immune–stromal interactions can predict the benefit of immunotherapy even in microsatellite-stable disease. The biomarker captures a complex interplay between immune infiltration and stromal architecture—features often underestimated by genomic profiling alone.
By transforming standard H&E slides into predictive, quantifiable datasets, this work illustrates how digital pathology and AI can refine patient selection for immunotherapy and accelerate the transition toward precision oncology in pMMR mCRC.
You can read the full abstract here.
The AtezoTRIBE and AVETRIC translational analyses show that AI-derived tumor microenvironment biomarkerscan identify subsets of pMMR colorectal cancer patients who benefit from ICI-based therapy. In AtezoTRIBE, biomarker-high status correlated with significantly longer PFS (p = 0.036) and OS (p = 0.024) under atezolizumab, and these findings were validated in AVETRIC (PFS p = 0.043; OS p = 0.053).

These results mark an important advance in AI-assisted immuno-oncology, suggesting that digital pathology could soon complement molecular testing in guiding treatment for colorectal cancer beyond MSI status.
LOS ANGELES – OCTOBER 17, 2025 – Netmarble, a renowned developer and publisher of high-quality games, is gearing up for the global launch of its ultimate dark fantasy MMORPG Raven2. The game will officially launch on October 22 at…

One of Harper Lee’s surviving relatives says it’s possible there could be major unpublished works by the author still to be discovered, following the release of eight of her previously unseen short stories.
Describing the mystery…

20 October 2025
Euro area current account balance
(EUR billions unless otherwise indicated; working day and seasonally adjusted data)
Source: ECB.
The current account of the euro area recorded a surplus of €12 billion in August 2025, a decrease of €18 billion from the previous month (Chart 1 and Table 1). Surpluses were recorded for goods (€15 billion) and services (€14 billion). Deficits were recorded for secondary income (€16 billion) and primary income (€1 billion).
Current account of the euro area
(EUR billions unless otherwise indicated; transactions; working day and seasonally adjusted data)
Source: ECB. Note: Discrepancies between totals and their components may be due to rounding.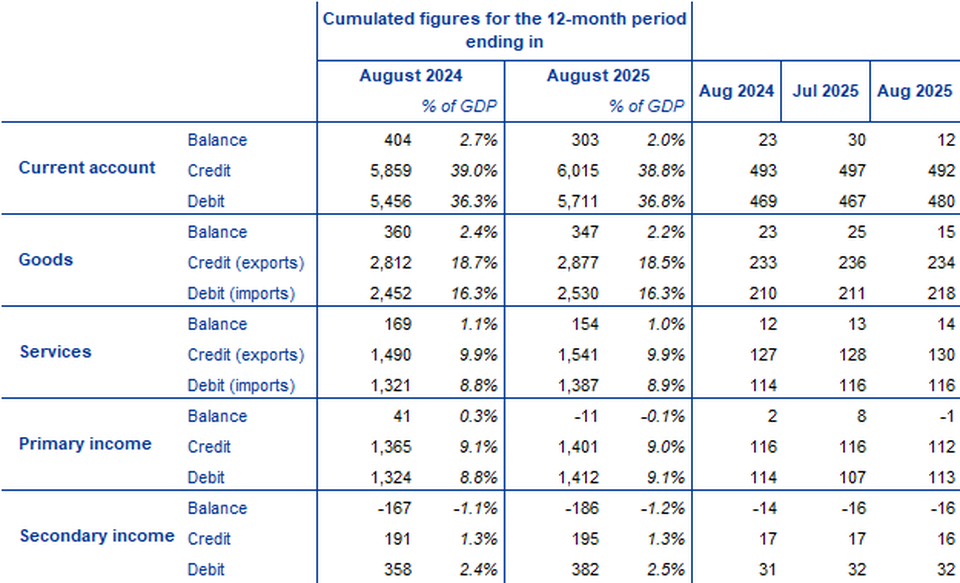
Data for the current account of the euro area
In the 12 months to August 2025, the current account recorded a surplus of €303 billion (2.0% of euro area GDP), compared with a surplus of €404 billion (2.7% of euro area GDP) one year earlier. This decrease was explained by a deterioration of all the accounts, particularly by a switch from a surplus (€41 billion) to a deficit (€11 billion) for primary income, but also by a larger deficit for secondary income (up from €167 billion to €186 billion), and reductions in the surplus for services (down from €169 billion to €154 billion) and goods (down from €360 billion to €347 billion).
Selected items of the euro area financial account
(EUR billions; 12-month cumulated data)
Source: ECB. Notes: For assets, a positive (negative) number indicates net purchases (sales) of non-euro area instruments by euro area investors. For liabilities, a positive (negative) number indicates net sales (purchases) of euro area instruments by non-euro area investors.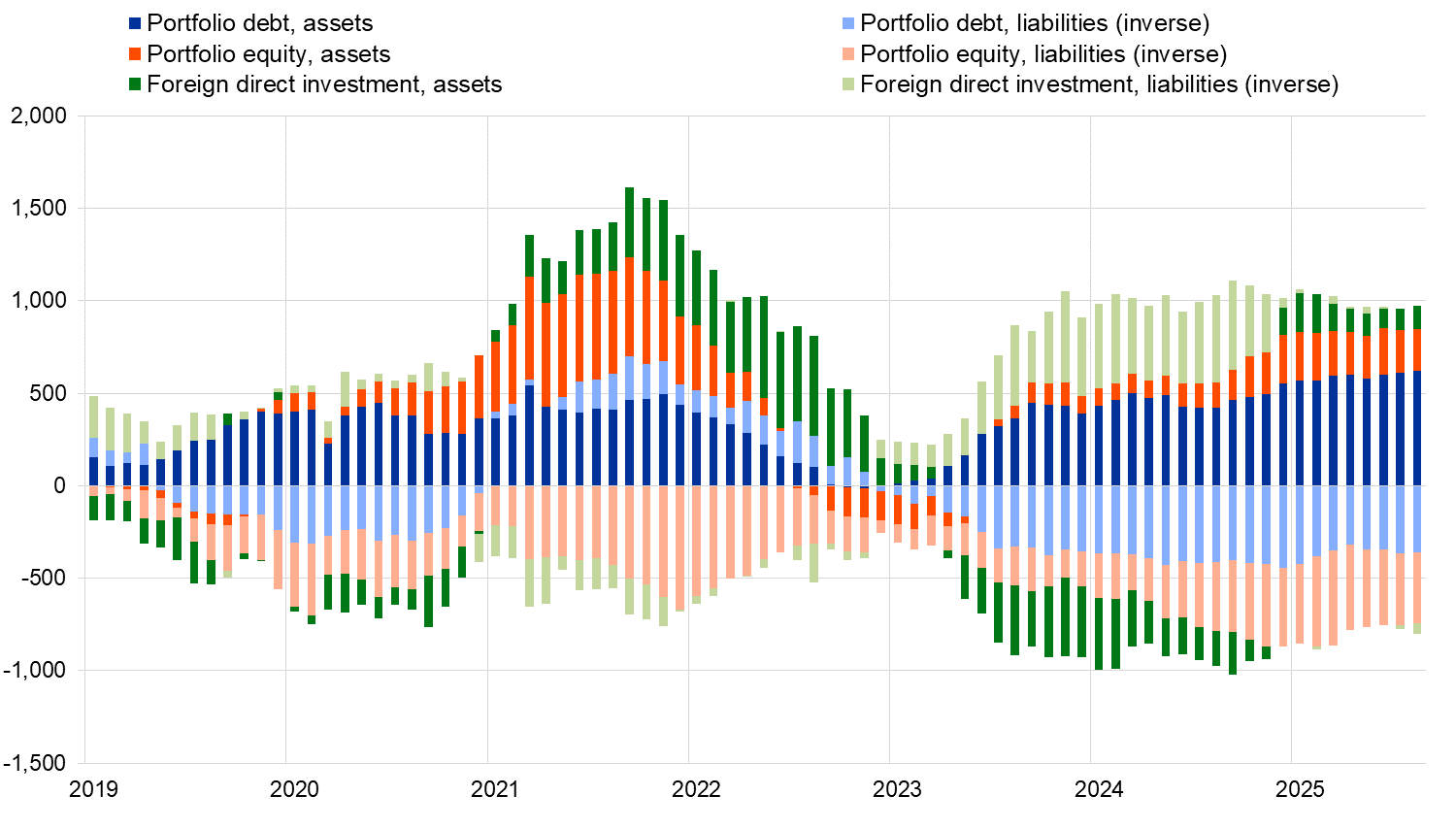
In direct investment, euro area residents made net investments of €123 billion in non-euro area assets in the 12 months to August 2025, following net disinvestments of €191 billion one year earlier (Chart 2 and Table 2). Non-residents invested €57 billion in net terms in euro area assets in the 12 months to August 2025, following net disinvestments of €472 billion one year earlier.
In portfolio investment, euro area residents’ net purchases of non-euro area equity increased to €226 billion in the 12 months to August 2025, up from €139 billion one year earlier. Over the same period, net purchases of non-euro area debt securities by euro area residents increased to €623 billion, up from €420 billion. Non-residents’ net purchases of euro area equity increased to €387 billion in the 12 months to August 2025, up from €370 billion one year earlier. Over the same period, non-residents made net purchases of euro area debt securities amounting to €359 billion, following net purchases of €415 billion.
Financial account of the euro area
(EUR billions unless otherwise indicated; transactions; non-working day and non-seasonally adjusted data)
Source: ECB. Notes: Decreases in assets and liabilities are shown with a minus sign. Net financial derivatives are reported under assets. “MFIs” stands for monetary financial institutions. Discrepancies between totals and their components may be due to rounding.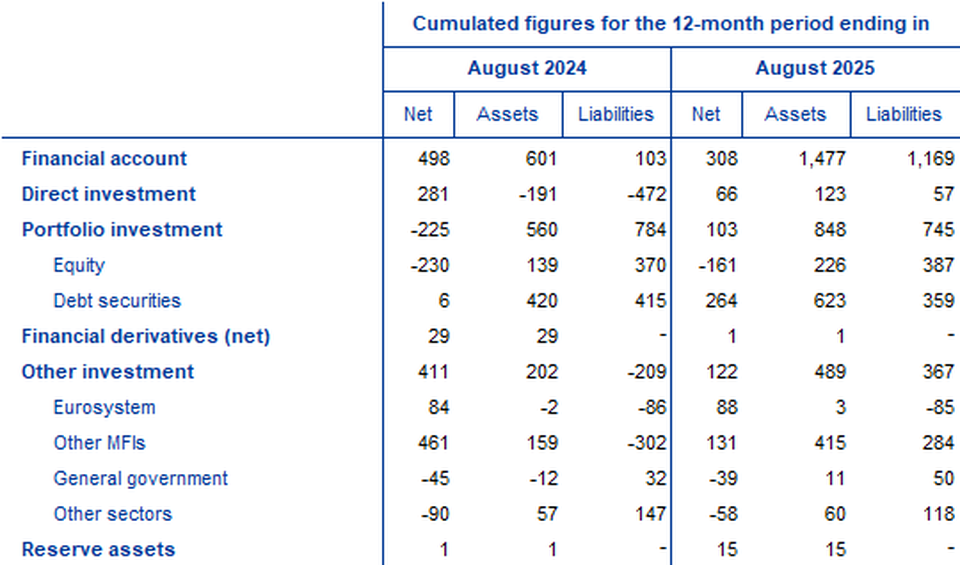
Data for the financial account of the euro area
In other investment, euro area residents recorded net acquisitions of non-euro area assets amounting to €489 billion in the 12 months to August 2025 (following net acquisitions of €202 billion one year earlier), while their net incurrence of liabilities was €367 billion (following net disposals of €209 billion one year earlier).
Monetary presentation of the balance of payments
(EUR billions; 12-month cumulated data)
Source: ECB. Notes: “MFI net external assets (enhanced)” incorporates an adjustment to the MFI net external assets (as reported in the consolidated MFI balance sheet items statistics) based on information on MFI long-term liabilities held by non-residents, available in b.o.p. statistics. B.o.p. transactions refer only to transactions of non-MFI residents of the euro area. Financial transactions are shown as liabilities net of assets. “Other” includes financial derivatives and statistical discrepancies.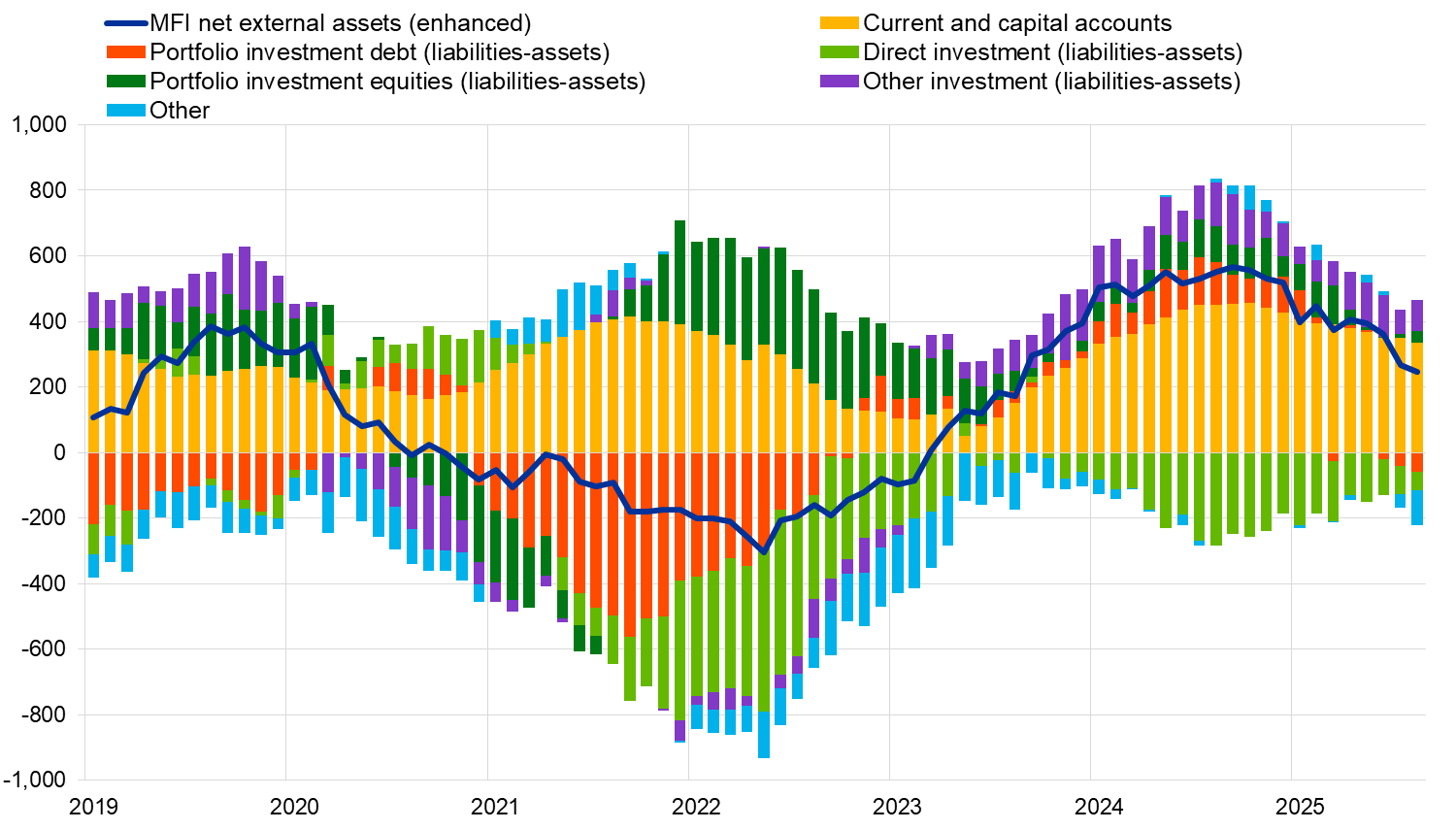
The monetary presentation of the balance of payments (Chart 3) shows that the net external assets (enhanced) of euro area MFIs increased by €245 billion in the 12 months to August 2025. This increase was driven by the current and capital accounts surplus and euro area non-MFIs’ net inflows in other investment and portfolio investment equity. These developments were partly offset by euro area non-MFIs’ net outflows in other flows, direct investment and portfolio investment debt.
In August 2025 the Eurosystem’s stock of reserve assets increased to €1,507.8 billion up from €1,499 billion in the previous month (Table 3). This increase was driven by positive price changes (€13.8 billion), mostly due to an increase in the price of gold, and, to a lesser extent, by net acquisitions of assets (€1.2 billion) which were partly offset by negative exchange rate changes (€6.2 billion).
Reserve assets of the euro area
(EUR billions; amounts outstanding at the end of the period, flows during the period; non-working day and non-seasonally adjusted data)
Source: ECB. Notes: “Other reserve assets” comprises currency and deposits, securities, financial derivatives (net) and other claims. Discrepancies between totals and their components may be due to rounding.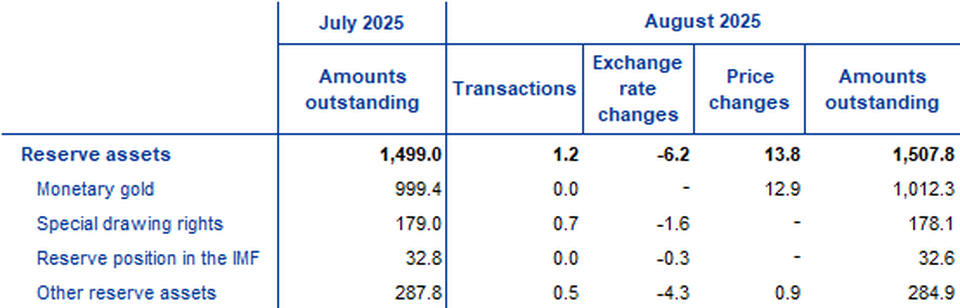
Data for the reserve assets of the euro area
Data revisions
This press release incorporates revisions to the data for July 2025. These revisions did not significantly alter the figures previously published.
Next releases:
For media queries, please contact Benoît Deeg, tel.: +49 172 1683704.

An experimental film should be approached in the same open-minded spirit in which it was created, but I must confess to being more or less defeated by this opaque, inert, micro-budget work from Canadian director Rhayne Vermette, who has worked…

The long-running legal battle between ValueLicensing and Microsoft over the resale of software licenses has taken another turn following Microsoft’s attempt to make the case about copyright.
The litigation has rumbled since 2021, beginning with…
Samsung Electronics has unveiled a complete redesign of Samsung Newsroom, its official online communications channel — transforming it into a visually driven platform centered on video, graphics and other types of…