Looking for the most recent regular Connections answers? Click here for today’s Connections hints, as well as our daily answers and hints for The New York Times Mini Crossword, Wordle and Strands puzzles.
Today’s Connections: Sports Edition is…

Looking for the most recent regular Connections answers? Click here for today’s Connections hints, as well as our daily answers and hints for The New York Times Mini Crossword, Wordle and Strands puzzles.
Today’s Connections: Sports Edition is…
This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Spotify has announced a redesigned version of its Apple TV app that the company says has been “rebuilt from the ground up for a faster, smarter, more visual experience.” The tvOS version of the Spotify app is also gaining several welcome…
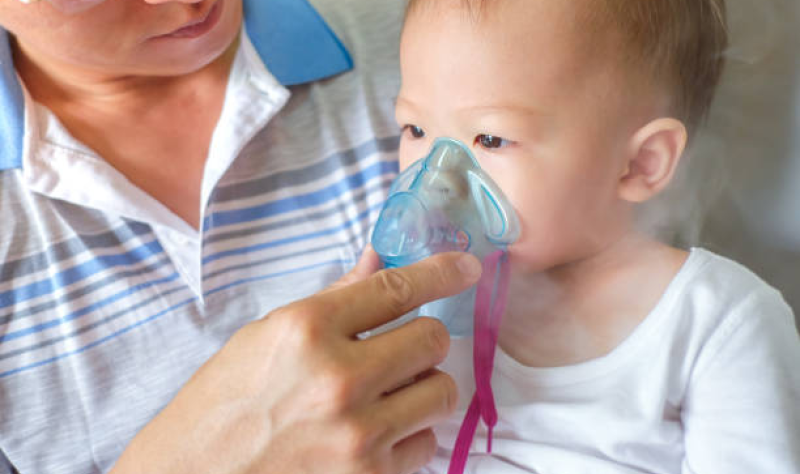
Two Dutch studies evaluating rates of respiratory syncytial virus (RSV) infection in younger and older age-groups reveal substantial primary-care burdens in both.
The adjusted pooled incidence of RSV among preschool…
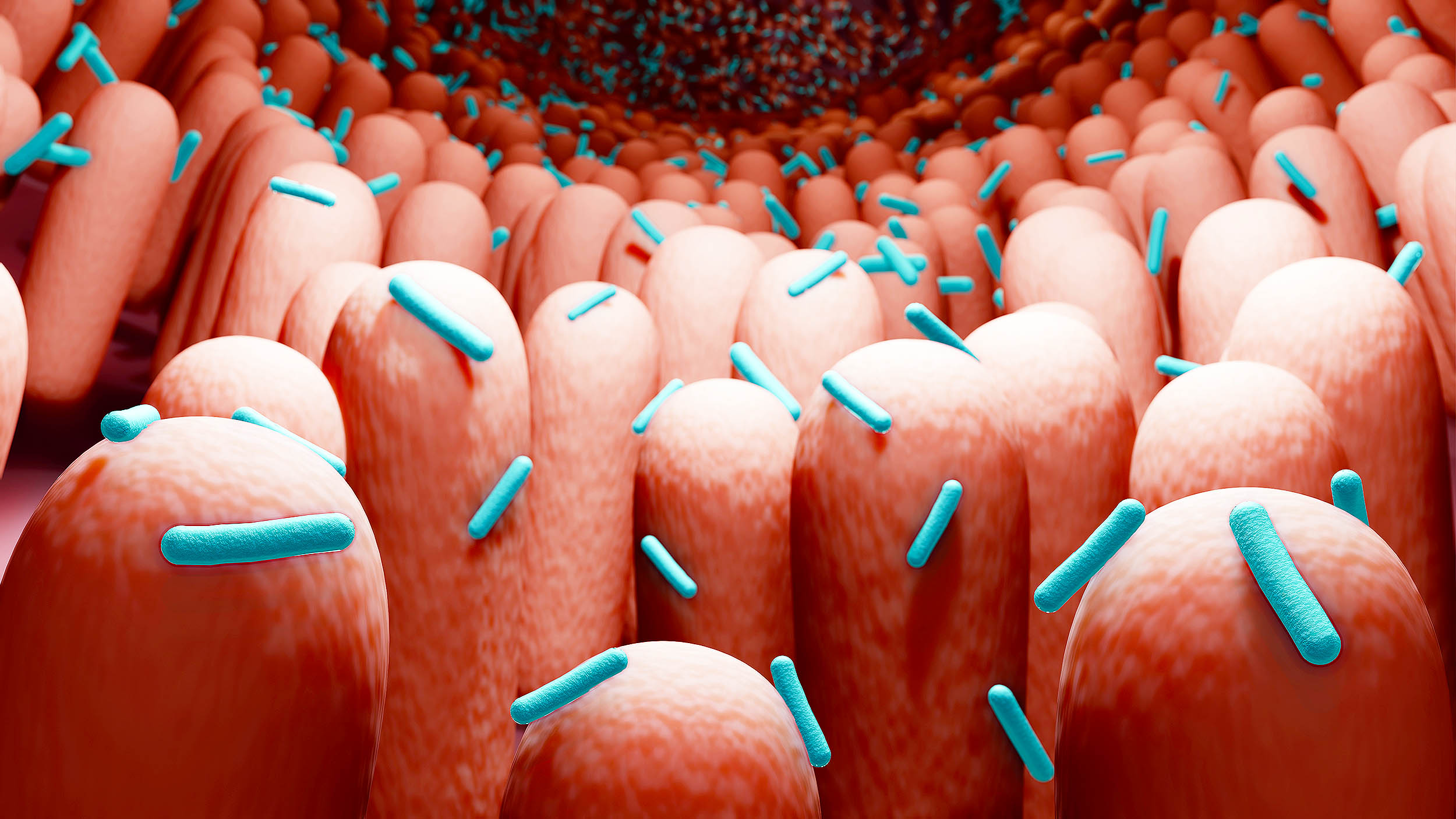
Humans carry around a busy community of microbes that help run daily life. This microbiome breaks down food, trains the immune system, and competes with germs.
Scientists just spotted something unexpected inside those communities: tiny circular
This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!

TOKYO (AP) — Japanese sushi legend Jiro Ono won three Michelin stars for more than a decade, the world’s oldest head chef to do so. He has served the world’s dignitaries and his art of sushi was featured in an…
The Lundquist Institute is proud to announce that Dong Chang, MD, MS has been awarded a prestigious R01 grant from the National Institutes of Health (NIH)/National Institute on Aging, totaling $3,163,459, for his groundbreaking…
Final analysis from the
The primary end point was progression-free survival (PFS), which was reached with statistically significant and clinically meaningful improvement. The median PFS was significantly longer in patients administered ITM-11 compared to those administered everolimus. The secondary end point of the trial was overall survival (OS), which was also identified to be higher in patients who were administered ITM-11 vs everolimus.2
There was a total of 207 patients in the ITM-11 group and 102 patients in the everolimus group. The median ages of both groups were 65 (ITM-11), and 61 (everolimus). Majority of patients in both groups were male. The majority of patients had grade 2, nonfunctional GEP-NETs and had received prior therapy.
COMPETE met its primary end point of PFS, which proved to be significantly longer in patients treated with ITM-11 vs everolimus. The central assessment was 23.9 vs 14.1 months (HR, 0.67; 95% CI, 0.48–0.95; P =.022).The local assessment was 24.1 vs 17.6 months (HR, 0.66; 95% CI, 0.48–0.91] P =.010;).
In the subgroup analysis of PFS by tumor origin, mPFS was found to be numerically longer in GE-NETs and P-NETs in the ITM-11 arm. In GE-NETs the mPFS was 23.9 vs 12 months (HR 0.64, 95% CI, 0.38–1.08; P =.090;). In P-NETs the mPFS was 24.5 vs 14.7 months (HR, 0.70, 95% CI, 0.45–1.09; P =.114;).
It was also identified that mPFS was numerically longer in grade 1 and significantly longer in grade 2 tumors in the ITM-11 arm. Grade 1 was 30 vs 23.7 months (HR, 0.89, 95% CI, 0.42–1.8; P =.753;), and grade 2 was 21.7 vs 9.2 months (HR 0.55l 95% CI, 0.37–0.82] P =.0003).
In exploring PFS by prior therapy, it was identified that mPFS was numerically longer in the first line and significantly longer in the second line in the ITM-11 arm. First line data showed the mPFS was not reached in the ITM-11 vs 18.1 months (HR, 0.60, 95% CI, 0.25–1.45; P =.249), and second line data showed 23.9 vs 14.1 months (HR, 0.68; 95% CI, 0.47–0,98] P=.039).
Overall response rates (ORR), one of the secondary end points of the trial, was found to be significantly higher in the ITM-11 arm. Central assessment was 21.9% vs 4.2% (P <.0001), and local assessment was 30.5% vs 8.4% (P <.0001).
Adverse events (AEs) related to the drug study were experienced by 82% of patients ITM-11 group and 97% of patients in the everolimus group. The most common AEs reported were nausea (30% vs 10.1%), diarrhea (14.3% vs 35.4%), asthenia (25.3% vs 31.3%), and fatigue (15.7% vs 15.2%). These AEs were expected based on the known safety profile of ITM-11.2
AEs leading to premature study discontinuation were 1.8% vs 15.2% among both groups, respectively, dose modification or discontinuation were 3.7% vs 52.5%, and patients with delayed study drug administration due to toxicity was 0.9% in the ITM-11 group and 0% in the everolimus group.2
Dosimetry data showed targeted tumor uptake with low exposure to healthy organs, with normal organ absorbed doses well below safety thresholds.
Patient inclusion criteria included being 18 or older, having well-differentiated, nonfunctional GE-NET or functional/nonfunctional P-NET; grade 1/2 unresectable or metastatic, progressive, SSRT-positive disease; and being treatment-naive to first-line therapies or progressing under prior second-line therapies.1,2
Morphologic imagining was conducted in 3-month intervals. The PFS follow-up was done every 3 months after the first 30 days. Long-term follow-up was done every 6 months.
“With these data combining extensive dosimetry information from more than 200 patients included in a prospective trial, ITM is laying the groundwork for improved therapeutic decision-making by providing important insights into tumor uptake and treatment variability,” Emmanuel Deshayes, MD, PhD, professor in biophysics and nuclear medicine at the Montpellier Cancer Institute in France, said in a news release.2 “It may offer clinically meaningful implications for optimizing individualized patient management.”
Dosimetry data from COMPETE shaped the design of ITM’s phase 3 COMPOSE (NCT04919226)4 trial with ITM-11 in well-differentiated, aggressive grade 2 or grade 3 SSTR-positive GEP-NET tumors, as well as the upcoming phase 1 pediatric KinLET (NCT06441331) study in SSTR-positive tumors.
DISCLOSURES: Capdevila noted grants and/or research support from Advanced Accelerator Applications, AstraZeneca, Amgen, Bayer, Eisai, Gilead, ITM, Novartis, Pfizer, and Roche; participation as a speaker, consultant, or advisor for Advanced Acclerator Applications, Advanz Pharma, Amgen, Bayer, Eisai, Esteve, Exelixis, Hutchmed, Ipsen, ITM, Lilly, Merck Serono, Novartis, Pfizer, Roche, and Sanofi; position as advisory board member for Amgen, Bayer, Eisai, Esteve, Exelixis, Ipsen, ITM, Lilly, Novartis, and Roche; and a leadership role and chair position for the Spanish Task Force for Neuroendocrine and Endocrine Tumours Group (GETNE).

Scientists at Indiana University have achieved a breakthrough in understanding the universe thanks to a collaboration between two major international experiments studying neutrinos, which are ubiquitous, tiny particles that stream…