The Lundquist Institute is proud to announce that Dong Chang, MD, MS has been awarded a prestigious R01 grant from the National Institutes of Health (NIH)/National Institute on Aging, totaling $3,163,459, for his groundbreaking…
Blog
-
COMPETE Trial: ITM-11 Tops Everolimus for GEP-NET PFS and OS | Targeted Oncology
Final analysis from the
phase 3 COMPETE trial (NCT03049189) demonstrated that ITM-11 (177Lu-edotretide) met its primary and secondary end points in patients with gastroenteropancreatic neuroendocrine tumors (GEP-NETs) compared with everolimus. Data were presented at the 2025 European Society for Medical Oncology (ESMO) Congress on October 18, 2025, by Jaume Capdevila, MD, PhD, Vall d’Hebron University Hospital, and at theNANETS Symposium on October 25.1The primary end point was progression-free survival (PFS), which was reached with statistically significant and clinically meaningful improvement. The median PFS was significantly longer in patients administered ITM-11 compared to those administered everolimus. The secondary end point of the trial was overall survival (OS), which was also identified to be higher in patients who were administered ITM-11 vs everolimus.2
There was a total of 207 patients in the ITM-11 group and 102 patients in the everolimus group. The median ages of both groups were 65 (ITM-11), and 61 (everolimus). Majority of patients in both groups were male. The majority of patients had grade 2, nonfunctional GEP-NETs and had received prior therapy.
COMPETE Trial Findings
COMPETE met its primary end point of PFS, which proved to be significantly longer in patients treated with ITM-11 vs everolimus. The central assessment was 23.9 vs 14.1 months (HR, 0.67; 95% CI, 0.48–0.95; P =.022).The local assessment was 24.1 vs 17.6 months (HR, 0.66; 95% CI, 0.48–0.91] P =.010;).
In the subgroup analysis of PFS by tumor origin, mPFS was found to be numerically longer in GE-NETs and P-NETs in the ITM-11 arm. In GE-NETs the mPFS was 23.9 vs 12 months (HR 0.64, 95% CI, 0.38–1.08; P =.090;). In P-NETs the mPFS was 24.5 vs 14.7 months (HR, 0.70, 95% CI, 0.45–1.09; P =.114;).
It was also identified that mPFS was numerically longer in grade 1 and significantly longer in grade 2 tumors in the ITM-11 arm. Grade 1 was 30 vs 23.7 months (HR, 0.89, 95% CI, 0.42–1.8; P =.753;), and grade 2 was 21.7 vs 9.2 months (HR 0.55l 95% CI, 0.37–0.82] P =.0003).
In exploring PFS by prior therapy, it was identified that mPFS was numerically longer in the first line and significantly longer in the second line in the ITM-11 arm. First line data showed the mPFS was not reached in the ITM-11 vs 18.1 months (HR, 0.60, 95% CI, 0.25–1.45; P =.249), and second line data showed 23.9 vs 14.1 months (HR, 0.68; 95% CI, 0.47–0,98] P=.039).
Overall response rates (ORR), one of the secondary end points of the trial, was found to be significantly higher in the ITM-11 arm. Central assessment was 21.9% vs 4.2% (P <.0001), and local assessment was 30.5% vs 8.4% (P <.0001).
Safety Profile
Adverse events (AEs) related to the drug study were experienced by 82% of patients ITM-11 group and 97% of patients in the everolimus group. The most common AEs reported were nausea (30% vs 10.1%), diarrhea (14.3% vs 35.4%), asthenia (25.3% vs 31.3%), and fatigue (15.7% vs 15.2%). These AEs were expected based on the known safety profile of ITM-11.2
AEs leading to premature study discontinuation were 1.8% vs 15.2% among both groups, respectively, dose modification or discontinuation were 3.7% vs 52.5%, and patients with delayed study drug administration due to toxicity was 0.9% in the ITM-11 group and 0% in the everolimus group.2
Dosimetry data showed targeted tumor uptake with low exposure to healthy organs, with normal organ absorbed doses well below safety thresholds.
Patient Characteristics
Patient inclusion criteria included being 18 or older, having well-differentiated, nonfunctional GE-NET or functional/nonfunctional P-NET; grade 1/2 unresectable or metastatic, progressive, SSRT-positive disease; and being treatment-naive to first-line therapies or progressing under prior second-line therapies.1,2
Morphologic imagining was conducted in 3-month intervals. The PFS follow-up was done every 3 months after the first 30 days. Long-term follow-up was done every 6 months.
“With these data combining extensive dosimetry information from more than 200 patients included in a prospective trial, ITM is laying the groundwork for improved therapeutic decision-making by providing important insights into tumor uptake and treatment variability,” Emmanuel Deshayes, MD, PhD, professor in biophysics and nuclear medicine at the Montpellier Cancer Institute in France, said in a news release.2 “It may offer clinically meaningful implications for optimizing individualized patient management.”
Dosimetry data from COMPETE shaped the design of ITM’s phase 3 COMPOSE (NCT04919226)4 trial with ITM-11 in well-differentiated, aggressive grade 2 or grade 3 SSTR-positive GEP-NET tumors, as well as the upcoming phase 1 pediatric KinLET (NCT06441331) study in SSTR-positive tumors.
DISCLOSURES: Capdevila noted grants and/or research support from Advanced Accelerator Applications, AstraZeneca, Amgen, Bayer, Eisai, Gilead, ITM, Novartis, Pfizer, and Roche; participation as a speaker, consultant, or advisor for Advanced Acclerator Applications, Advanz Pharma, Amgen, Bayer, Eisai, Esteve, Exelixis, Hutchmed, Ipsen, ITM, Lilly, Merck Serono, Novartis, Pfizer, Roche, and Sanofi; position as advisory board member for Amgen, Bayer, Eisai, Esteve, Exelixis, Ipsen, ITM, Lilly, Novartis, and Roche; and a leadership role and chair position for the Spanish Task Force for Neuroendocrine and Endocrine Tumours Group (GETNE).
REFERENCES:
1. Capdevilla J, Amthauer H, Ansquer C, et al. Efficacy, safety and subgroup analysis of 177Lu-edotreotide vs everolimus in patients with grade 1 or grade 2 GEP-NETs: Phase 3 COMPETE trial. Presented at: 2025 ESMO Congress; October 17-20, 2025; Berlin, Germany. Abstract 1706O
2. ITM presents dosimetry data from phase 3 COMPETE trial supporting favorable efficacy and safety profile with n.c.a. 177Lu-edotreotide (ITM-11) in patients with gastroenteropancreatic neuroendocrine tumors at EANM 2025 Annual Congress. News release. ITM. October 8, 2025. Accessed October 18, 2025.
https://tinyurl.com/3nuscs4m 3. Lutetium 177Lu-Edotreotide versus best standard of care in well-differentiated aggressive grade-2 and grade-3 gastroenteropancreatic neuroendocrine tumors (GEP-NETs) – COMPOSE (COMPOSE). ClinicalTrials.gov. Updated September 10, 2025. Accessed October 18, 2025.
https://www.clinicaltrials.gov/study/NCT04919226 4. Phase I trial to determine the dose and evaluate the PK and safety of lutetium Lu 177 edotreotide therapy in pediatric participants with SSTR-positive tumors (KinLET). ClinicalTrials.gov. Updated September 19, 2025. Accessed October 18, 2025.
https://www.clinicaltrials.gov/study/NCT06441331 Continue Reading
-

OnePlus 15 Goes on Sale in China, and Its Global Launch Appears Imminent
The OnePlus 15 is now available to purchase in China, and it’s likely that a global model that would arrive in the US and the UK will be announced soon.
The Chinese edition of the phone comes in three colors: the previously announced Sand Storm…
Continue Reading
-

Imogen Poots to Receive Denver Film’s Excellence in Acting Award
Imogen Poots will be swimming her way to honors from the Denver Film Festival.
The star of Kristen Stewart’s feature directorial debut The Chronology of Water has been selected to receive an excellence in acting award…
Continue Reading
-
Liver transplants from MAiD donors show outcomes comparable to standard donations
Organ donation following medical assistance in dying (MAiD), also known as euthanasia, is a relatively new practice both in North America and worldwide. A first comparison of liver transplantation using organs donated after MAiD in…
Continue Reading
-

O’Brien Confident as Ireland Prepare for All Blacks Rematch in Chicago » allblacks.com
Ireland wing Tommy O’Brien said ahead of their Test in Chicago on Sunday (NZT) that while the All Blacks have their respect, they are no longer on a pedestal.
Since breaking their duck against the New Zealanders in their 2016…
Continue Reading
-
UBC Study Unveils Why Honey Bees Dethrone Queens
It sounds like the plot of a medieval historical drama: A once-powerful monarch, weakened by illness, is overthrown by her previously loyal subjects. But in honey bee colonies, such high-stakes coups aren’t just fantasy — they’re a…
Continue Reading
-

Promoting Co-Benefit Actions for Positive Environmental and Social Impacts from Renewables – News
Speakers from financial institutions, NGOs, government, and industry highlighted how biodiversity protection, community engagement, and energy development can be mutually reinforcing when built into project design and policy frameworks.
Examples ranged from solar projects in France that integrate wetland restoration, eco-grazing, and citizen investment, to marine wind farms in the North Sea linked to long-term marine conservation funding. In Uzbekistan, solar developers are protecting tortoise habitats and partnering with local herders to manage grazing. In Qinghai Province, China, large-scale PV parks are reversing desertification while supporting ecological animal husbandry.
Industry actors like TotalEnergies are scaling agro-photovoltaic models that combine renewable energy generation with sustainable farming. Policymakers and financial institutions, including the European Bank for Reconstruction and Development (EBRD) and the International Renewable Energy Agency (IRENA), underscored the role of strong policy frameworks, financing incentives, and capacity building to scale these approaches globally.
Ecowende is developing the Netherlands’ most ecological offshore wind farm to date—powering 3% of national demand while enhancing North Sea biodiversity—through an innovative, research-driven and collaborative approach supported by IUCN’s Biodiversity Advisory Team, which provides independent review and recommendations on biodiversity goals and targets.
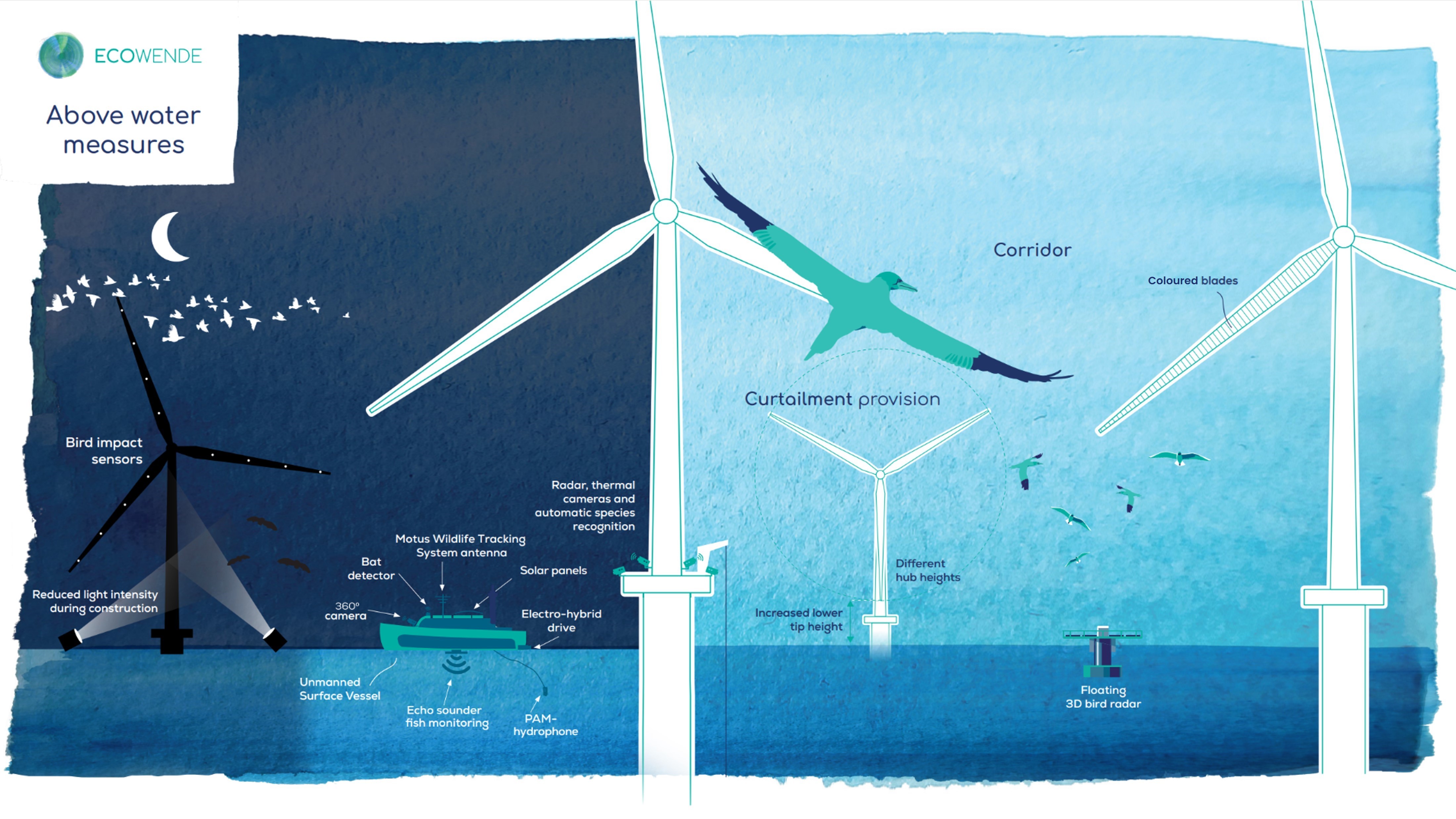
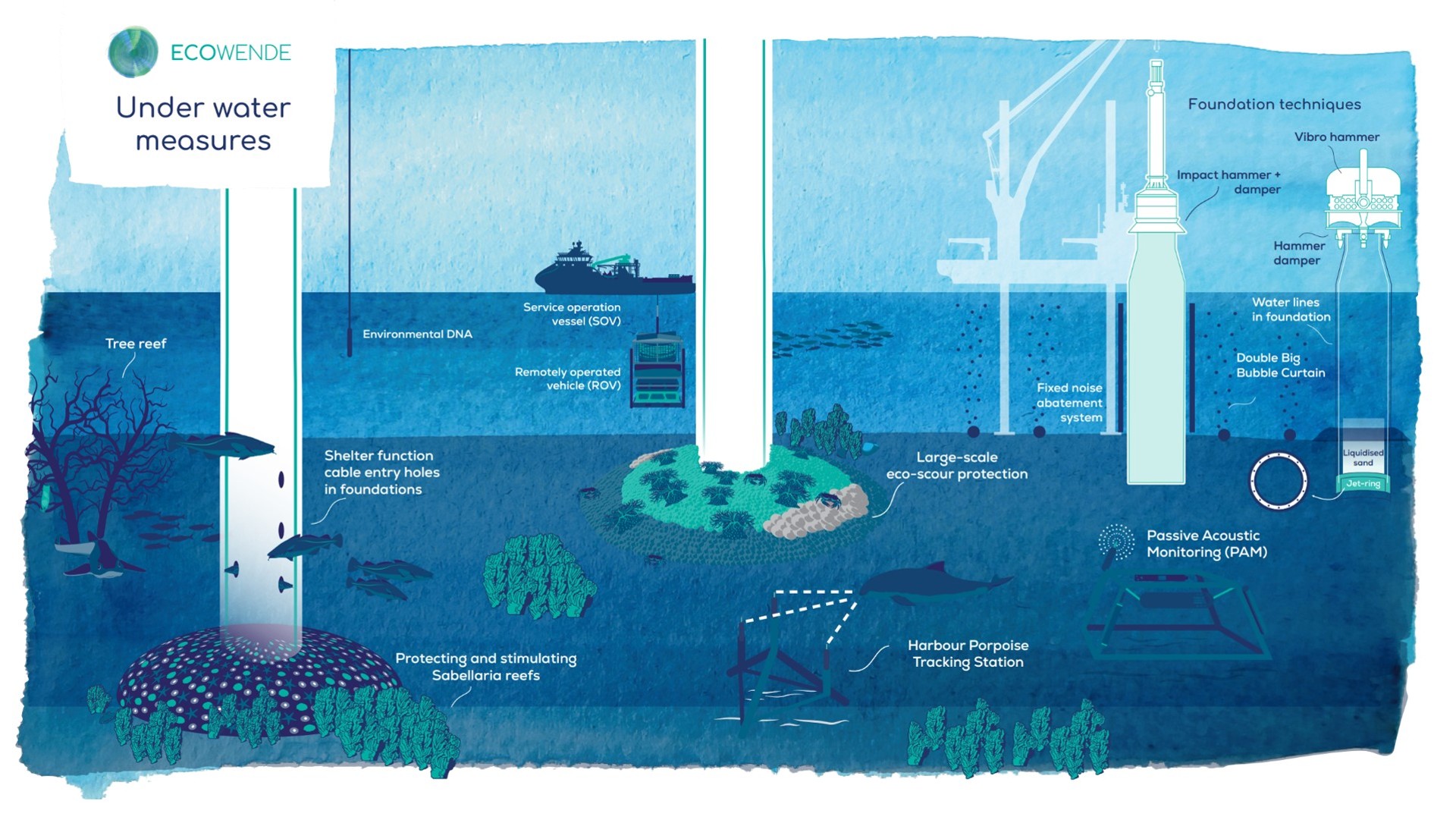
From regulatory tools to community partnerships, the session highlighted that the energy transition can—and must—deliver measurable gains for biodiversity and people.
The session was moderated by Qiulin Liu from IUCN and speakers included Adonai Herrera-Martínez from EBRD, Aonghais Cook from The Biodiversity Consultancy, Dr. Ma Hao from the Qinghai Provincial Development and Reform Commission, Jinlei Feng from IRENA, Karen Westley from Ipieca, Libby Sandbrook from Fauna & Flora, Sophie Depraz from Ipieca, Steven Dickinson from TotalEnergies, Yu Miao from SPIC Huanghe Hydropower Development Co., Ltd., and Zhang Jiali from the China Renewable Energy Engineering Institute (CREEI).
Continue Reading
-

Josh Hartnett To Star In Tommy Wirkola Film ‘All Day & All Night’
EXCLUSIVE: Josh Hartnett (Trap) will star in and produce the action-thriller All Day & All Night, written by Tommy Wirkola and John Niven and to be directed by Wirkola, the Norwegian filmmaker best known for the Violent Night and Dead Snow…
Continue Reading
-
Just a moment…
Just a moment… This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Continue Reading