Lindsey Vonn on going for medals at Milano Cortina 2026: ‘I like it when the stakes are high’
While the 2024/25 season was largely about proving herself in her comeback, Vonn did so in dramatic fashion, finishing the World Cup season with a…

While the 2024/25 season was largely about proving herself in her comeback, Vonn did so in dramatic fashion, finishing the World Cup season with a…
This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
As the incidence of obesity continues to rise nationally and globally, a new observational study published in Cancer reports that liver recurrence and survival outcomes in patients with pancreatic adenocarcinoma (PDAC) after upfront surgical…
A study published in the Annals of Internal Medicine looks at one long walk vs multiple short walks and…

OLED TVs are considered the best TVs for gaming, but they can also be very expensive. Fortunately, LG has a B-series OLED lineup that’s hundreds of dollars cheaper than its other OLED TVs while still offering incredible image quality and gaming…
Some of this year’s early Black Friday deals are already live, featuring savings on just about every category you can think of—including virtual private networks (VPNs). With NordVPN’s early offers, for instance, you can save up to 77% on a…

Staud threw open the doors to its new Melrose Avenue flagship in Los Angeles last week, unveiling the boutique alongside the debut of its holiday 2025 collection.
Cofounders Sarah Staudinger and George Augusto hosted friends…

After several years of close collaboration and a relationship rooted in mutual respect and shared vision, Fogarty Innovation is coming together with the Cardiovascular Research Foundation® (CRF®) to create a unified platform for advancing transformative healthcare technologies. This strategic combination strengthens CRF’s leadership in medtech by integrating Fogarty’s renowned expertise in early-stage innovation, creating a powerful, cross-specialty platform to accelerate transformative breakthroughs into patient care. The merger was announced during a special keynote session at the Transcatheter Cardiovascular Therapeutics® (TCT®) meeting.
This platform unites two mission-driven organizations with a shared vision: to catalyze the next generation of disruptive medical technologies by advancing innovations that have the power to transform patient care and reshape the future of healthcare. It builds on a strong track record of successful collaboration: CRF and Fogarty Innovation have partnered on the TCT MedTech Innovation Forum over the past four years, fostering early-stage innovation and supporting emerging medtech entrepreneurs. Together, they form a powerful alliance poised to accelerate progress in cardiovascular medicine and transform patient care on a global scale.
The unified platform will unlock immediate access to world-class incubation, long-term strategic growth, and enhanced philanthropic impact. By combining the deep expertise of both organizations, it will provide unparalleled access to seasoned medtech executives, expand innovation education initiatives, and increase business opportunities in Silicon Valley and beyond – all while accelerating the development of cutting-edge technologies and driving measurable improvements in patient care.
Fogarty Innovation will continue to carry its name and mission, now serving as CRF’s West Coast innovation hub. Together, CRF and Fogarty Innovation will amplify their collective impact by combining resources, expertise, and leadership – aligning on strategic initiatives to drive innovation and expand their reach across the cardiovascular landscape.
“We’re thrilled to enter into this partnership with our close friends and colleagues at Fogarty Innovation,” said Juan F. Granada, MD, President and Chief Executive Officer of CRF. “We are entering a groundbreaking era in cardiovascular medicine; one defined by unprecedented technological potential. This move is not just a step forward; it is a bold move to lead the future. By uniting our strengths into a single, purpose-driven platform, we are shaping the development of transformative technologies that will redefine care and bring us closer to a more equitable health care system.”
Over the past 17 years, Fogarty Innovation has demonstrated that our model of immersive support – through incubation, acceleration, education, and alliances – meaningfully increases the success of innovators in bringing new tools and therapies to clinicians and patients. We are excited to join forces with CRF, as we can now scale that impact globally, giving entrepreneurs a larger stage, stronger resources, and a faster path to delivering transformative care.”
Andrew Cleeland, CEO of Fogarty Innovation
“Our partnerships have always been rooted in trust, shared purpose, the highest ethical standards, performance excellence, and a commitment to transform the lives of patients everywhere,” said Martin B. Leon, MD, Founder and Chairman Emeritus of CRF. “CRF’s mission to advance cardiovascular care through research and education will be amplified by Fogarty Innovation, opening new opportunities for collaborations, advanced innovation, and strategic growth – ultimately, impacting the science and practice of medicine worldwide.”
Source:
Cardiovascular Research Foundation
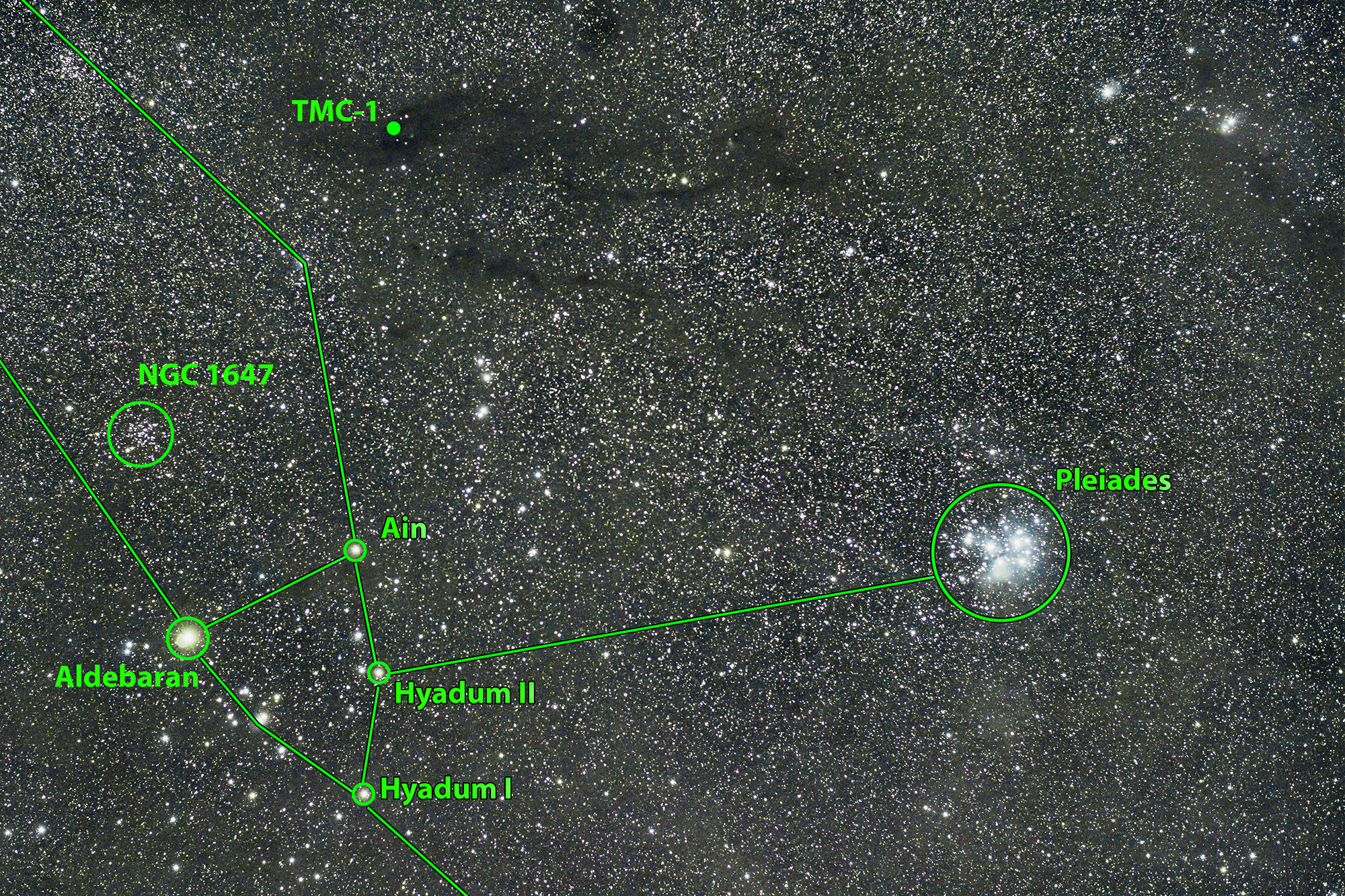
MIT researchers recently studied a region of space called the Taurus Molecular Cloud-1 (TMC-1) and discovered more than 100 different molecules floating in the gas there — more than in any other known interstellar…
New study results from a large international all-comer population of percutaneous coronary intervention (PCI) candidates found that utilizing a strategy of sirolimus-eluting balloons with bailout stenting only if necessary was noninferior to routine drug-eluting stent (DES) implantation as part of the treatment for de novo coronary artery disease.
Findings were reported today at TCT® 2025, the annual scientific symposium of the Cardiovascular Research Foundation® (CRF®). TCT is the world’s premier educational meeting specializing in interventional cardiovascular medicine.
DES are implanted in the vast majority of PCIs with well-established immediate and mid-term outcomes. However, long term follow-up studies have reported annual adverse event rates of 2-4%. This has led to a growing interest in strategies that minimize metallic stent implantation to potentially reduce late events. The study used the SELUTION SLR Drug-Eluting Balloon (DEB) which delivers a sustained drug release maintaining therapeutic tissue concentration for up to 90 days designed to have a similar elution profile to current DES.
Between August 2021 and July 2024, a total of 3,341 participants were randomized one to one to either the DEB (n=1,671) or DES strategy (n=1,670) at 62 sites in 12 countries across Europe and Asia. Patients with lesion reference vessel diameter (RVD) ≥2.0 and ≤5.0 mm were eligible for inclusion. Baseline characteristics were similar in both groups, with a relatively high proportion of patients presenting with acute coronary syndromes or having high bleeding risk. Randomization occurred after angiography when all target lesions were considered suitable for either strategy and prior to lesion wiring and lesion preparation. Eighty percent of participants treated with the SEB did not require a stent.
The primary endpoint of target vessel failure, comprised of cardiac death, target vessel-related myocardial infarction and clinically driven target vessel revascularization, occurred in 5.3% of the DEB strategy group and 4.4% of the DES strategy group at one year (Risk Difference 0.91, 95% CI: -0.55, 2.38%, Pnoninferiority=0.02). No acute or late safety concerns were noted with the DEB strategy, with low rates of cardiac death (0.70% vs 1.0%), lesion thrombosis (0.1 %vs 0.3%), and target vessel myocardial infarction (2.7% vs 2.6%) comparable with DES.
The SELUTION DeNovo trial provides the first comparison of a PCI strategy based on the use of sirolimus-eluting balloons versus systematic implantation of DES in a large international all-comer population of PCI candidates. With no acute or late safety concerns, these results apply to a significant segment of PCI procedures including high-risk patients and complex lesions. We look forward to obtaining five-year data to determine long-term noninferiority or possible superiority of this strategy.”
Christian M. Spaulding, MD, PhD, Chief, Integrated Interventional Laboratory at European Hospital Georges Pompidou in Paris, France
The study was funded by M.A. Med Alliance SA (a Cordis Company), Switzerland.
Dr. Spaulding reported receiving grant /research support from the French Ministry of Health, CERC; consultant fees / honoraria from Medtronic, Techwald, Sanofi, Novartis Sonivie, Valcare and Boston Scientific as well as individual stock(s)/stock options/salary support from Cordis (MedAlliance) and Sonivie.
The results of the study were presented on Sunday, October 26, 2025, at 11:00 a.m. PT in the Main Arena (Hall A, Exhibition Level, Moscone South) at the Moscone Center during TCT 2025.
Source:
Cardiovascular Research Foundation