Mahmoud Shakshak, right, holds the bodies of his 5-year-old son, Fadi, and his 8-year-old daughter, Sara, who were killed in an Israeli army strike, at Shifa Hospital in Gaza City Wednesday, Oct. 29, 2025. (AP Photo/Yousef Al Zanoun)
After a…
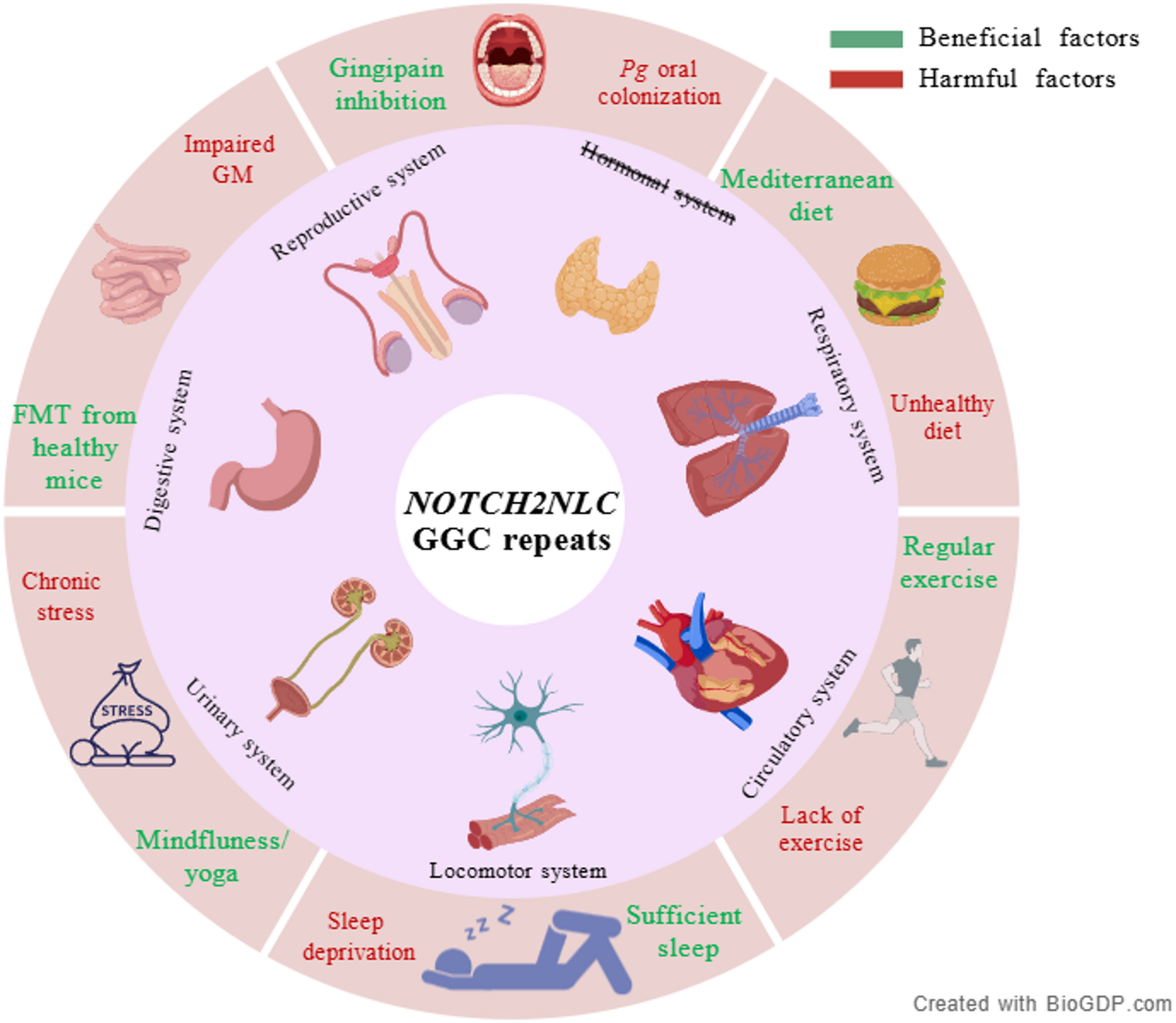
Sung JH, et al. An unusual degenerative disorder of neurons associated with a novel intranuclear hyaline inclusion (neuronal intranuclear hyaline inclusion disease). A clinicopathological study of a case. J Neuropathol Exp Neurol. 1980;39(2):107–30.
Google Scholar
Sone J, et al. Clinicopathological features of adult-onset neuronal intranuclear inclusion disease. Brain. 2016;139(Pt 12):3170–86.
Google Scholar
Sone J, et al. Long-read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat Genet. 2019;51(8):1215–21.
Google Scholar
Tian Y, et al. Expansion of human-specific GGC repeat in neuronal intranuclear inclusion disease-related disorders. Am J Hum Genet. 2019;105(1):166–76.
Google Scholar
Sun QY, et al. Expansion of GGC repeat in the human-specific NOTCH2NLC gene is associated with essential tremor. Brain. 2020;143(1):222–33.
Google Scholar
Ehrlich ME, Ellerby LM. Neuronal intranuclear inclusion disease: polyglycine protein is the culprit. Neuron. 2021;109(11):1757–60.
Google Scholar
Yu J, et al. CGG repeat expansion in NOTCH2NLC causes mitochondrial dysfunction and progressive neurodegeneration in drosophila model. Proc Natl Acad Sci U S A. 2022;119(41):e2208649119.
Google Scholar
Liu Q, et al. Expression of expanded GGC repeats within NOTCH2NLC causes behavioral deficits and neurodegeneration in a mouse model of neuronal intranuclear inclusion disease. Sci Adv. 2022;8(47):eadd6391.
Google Scholar
Zhong S, et al. Upstream open reading frame with NOTCH2NLC GGC expansion generates polyglycine aggregates and disrupts nucleocytoplasmic transport: implications for polyglycine diseases. Acta Neuropathol. 2021;142(6):1003–23.
Google Scholar
Fan Y, et al. GGC repeat expansion in NOTCH2NLC induces dysfunction in ribosome biogenesis and translation. Brain. 2023;146(8):3373–91.
Google Scholar
Tian Y, et al. Clinical features of NOTCH2NLC-related neuronal intranuclear inclusion disease. J Neurol Neurosurg Psychiatry. 2022;93(12):1289–98.
Google Scholar
Chen H, et al. Re-defining the clinicopathological spectrum of neuronal intranuclear inclusion disease. Ann Clin Transl Neurol. 2020;7(10):1930–41.
Google Scholar
Tai H, et al. Clinical features and classification of neuronal intranuclear inclusion disease. Neurol Genet. 2023;9(2):e200057.
Google Scholar
Jiang S, et al. Generic diagramming platform (GDP): a comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2025;53(D1):D1670–6.
Google Scholar
Pang J, et al. The value of NOTCH2NLC gene detection and skin biopsy in the diagnosis of neuronal intranuclear inclusion disease. Front Neurol. 2021;12:624321.
Google Scholar
Lee HG, et al. Neuroinflammation: an astrocyte perspective. Sci Transl Med. 2023;15(721):eadi7828.
Google Scholar
Bao L et al. Immune system involvement in neuronal intranuclear inclusion disease. Neuropathol Appl Neurobiol, 2024;50(2):e12976.
Mori K, et al. Imaging findings and pathological correlations of subacute encephalopathy with neuronal intranuclear inclusion disease-case report. Radiol Case Rep. 2022;17(12):4481–6.
Google Scholar
Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci. 2017;20(2):136–44.
Google Scholar
Yoshii D, et al. An autopsy case of adult-onset neuronal intranuclear inclusion disease with perivascular preservation in cerebral white matter. Neuropathology. 2021;42(1):66–73.
Google Scholar
Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503.
Google Scholar
Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117(14):3720–32.
Google Scholar
Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295.
Google Scholar
Lu X, Hong D. Neuronal intranuclear inclusion disease: recognition and update. J Neural Transm. 2021;128(3):295–303.
Google Scholar
Bao L, et al. Utility of labial salivary gland biopsy in the histological diagnosis of neuronal intranuclear inclusion disease. Eur J Neurol. 2024;31(1):e16102.
Google Scholar
Zhong S, et al. Spatial and Temporal distribution of white matter lesions in NOTCH2NLC-Related neuronal intranuclear inclusion disease. Neurology. 2025;104(4):e213360.
Google Scholar
Du N et al. Value of neutrophil-to-lymphocyte ratio in neuronal intranuclear inclusion disease. Heliyon, 2024;10(6):e27953.
Yan Y, et al. The clinical characteristics of neuronal intranuclear inclusion disease and its relation with inflammation. Neurol Sci. 2023;44(9):3189–97.
Google Scholar
Shen Y et al. uN2CpolyG-mediated p65 nuclear sequestration suppresses the NF-κB-NLRP3 pathway in neuronal intranuclear inclusion disease. Cell Communication Signal, 2025;23(1):68.
Tateishi J, et al. Intranuclear inclusions in muscle, nervous tissue, and adrenal gland. Acta Neuropathol. 1984;63(1):24–32.
Google Scholar
Platero JL et al. The impact of coconut oil and Epigallocatechin gallate on the levels of IL-6, anxiety and disability in multiple sclerosis patients. Nutrients, 2020;12(2):305.
Casali BT, et al. Omega-3 fatty acids augment the actions of nuclear receptor agonists in a mouse model of alzheimer’s disease. J Neurosci. 2015;35(24):9173–81.
Google Scholar
Chauhan A, et al. Phytochemicals targeting NF-κB signaling: potential anti-cancer interventions. J Pharm Anal. 2022;12(3):394–405.
Google Scholar
Shabab T, et al. Neuroinflammation pathways: a general review. Int J Neurosci. 2017;127(7):624–33.
Google Scholar
Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651.
Google Scholar
Gleeson M, et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–15.
Google Scholar
Pedersen BK. Adolph distinguished lecture: muscle as an endocrine organ: IL-6 and other myokines. J Appl Physiol (1985). 2009;107(4):1006–14. Edward F.
Google Scholar
Steensberg A, et al. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285(2):E433–7.
Google Scholar
Ma Q. Beneficial effects of moderate voluntary physical exercise and its biological mechanisms on brain health. Neurosci Bull. 2008;24(4):265–70.
Google Scholar
Adlard PA, et al. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25(17):4217–21.
Google Scholar
Periasamy S, et al. Sleep deprivation-induced multi-organ injury: role of oxidative stress and inflammation. Excli J. 2015;14:672–83.
Google Scholar
Shearer WT, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107(1):165–70.
Google Scholar
Ooms S, et al. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 2014;71(8):971–7.
Google Scholar
Reiter RJ, et al. Brain washing and neural health: role of age, sleep, and the cerebrospinal fluid melatonin rhythm. Cell Mol Life Sci. 2023;80(4):88.
Google Scholar
Zhao HY, et al. Chronic sleep restriction induces cognitive deficits and cortical Beta-Amyloid deposition in mice via BACE1-Antisense activation. CNS Neurosci Ther. 2017;23(3):233–40.
Google Scholar
Zhang Y, et al. Quercetin ameliorates memory impairment by inhibiting abnormal microglial activation in a mouse model of Paradoxical sleep deprivation. Biochem Biophys Res Commun. 2022;632:10–6.
Google Scholar
Irwin MR, et al. Tai Chi compared with cognitive behavioral therapy and the reversal of systemic, cellular and genomic markers of inflammation in breast cancer survivors with insomnia: A randomized clinical trial. Brain Behav Immun. 2024;120:159–66.
Google Scholar
Dutcher JM, et al. Smartphone mindfulness meditation training reduces Pro-inflammatory gene expression in stressed adults: A randomized controlled trial. Brain Behav Immun. 2022;103:171–7.
Google Scholar
Walsh E, Eisenlohr-Moul T, Baer R. Brief mindfulness training reduces salivary IL-6 and TNF-α in young women with depressive symptomatology. J Consult Clin Psychol. 2016;84(10):887–97.
Google Scholar
Patel DI, et al. Therapeutic yoga reduces pro-tumorigenic cytokines in cancer survivors. Support Care Cancer. 2022;31(1):33.
Google Scholar
Streeter CC, et al. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses. 2012;78(5):571–9.
Google Scholar
Ozturk FO, Tezel A. Effect of laughter yoga on mental symptoms and salivary cortisol levels in first-year nursing students: A randomized controlled trial. Int J Nurs Pract. 2021;27(2):e12924.
Google Scholar
Nair RG, Vasudev MM, Mavathur R. Role of yoga and its plausible mechanism in the mitigation of DNA damage in Type-2 diabetes: A randomized clinical trial. Ann Behav Med. 2022;56(3):235–44.
Google Scholar
Chen SJ, et al. Association of fecal and plasma levels of Short-Chain fatty acids with gut microbiota and clinical severity in patients with Parkinson disease. Neurology. 2022;98(8):e848–58.
Google Scholar
Chakraborty P, Gamage H, Laird AS. Butyrate as a potential therapeutic agent for neurodegenerative disorders. Neurochem Int. 2024;176:105745.
Google Scholar
Li N, et al. Prebiotic inulin controls Th17 cells mediated central nervous system autoimmunity through modulating the gut microbiota and short chain fatty acids. Gut Microbes. 2024;16(1):2402547.
Google Scholar
Zhang Y, et al. Transmission of alzheimer’s disease-associated microbiota dysbiosis and its impact on cognitive function: evidence from mice and patients. Mol Psychiatry. 2023;28(10):4421–37.
Google Scholar
Kim MS, et al. Transfer of a healthy microbiota reduces amyloid and Tau pathology in an alzheimer’s disease animal model. Gut. 2020;69(2):283–94.
Google Scholar
Beydoun MA, et al. Clinical and bacterial markers of periodontitis and their association with incident All-Cause and alzheimer’s disease dementia in a large National survey. J Alzheimers Dis. 2020;75(1):157–72.
Google Scholar
Ilievski V, et al. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLoS ONE. 2018;13(10):e0204941.
Google Scholar
Wang RP, et al. IL-1β and TNF-α play an important role in modulating the risk of periodontitis and alzheimer’s disease. J Neuroinflammation. 2023;20(1):71.
Google Scholar
Dominy SS, et al. Porphyromonas gingivalis in alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5(1):eaau3333.
Google Scholar
Brettschneider J, et al. Microglial activation correlates with disease progression and upper motor neuron clinical symptoms in amyotrophic lateral sclerosis. PLoS ONE. 2012;7(6):e39216.
Google Scholar
Graves MC, et al. Inflammation in amyotrophic lateral sclerosis spinal cord and brain is mediated by activated macrophages, mast cells and T cells. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5(4):213–9.
Google Scholar
Henkel JS, et al. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2004;55(2):221–35.
Google Scholar
Liu YH, et al. Neuronal intranuclear inclusion disease in patients with adult-onset non-vascular leukoencephalopathy. Brain. 2022;145(9):3010–21.
Google Scholar
Chen L, et al. Teaching neuroimages: the zigzag edging sign of adult-onset neuronal intranuclear inclusion disease. Neurology. 2019;92(19):e2295-6.
Google Scholar
Fragoso DC, et al. Imaging of Creutzfeldt-Jakob disease: imaging patterns and their differential diagnosis. Radiographics. 2017;37(1):234–57.
Google Scholar
Muttikkal TJ, Wintermark M. MRI patterns of global hypoxic-ischemic injury in adults. J Neuroradiol. 2013;40(3):164–71.
Google Scholar
Hasebe M, et al. Hypoglycemic encephalopathy. QJM. 2022;115(7):478–9.
Google Scholar
Zhang Z, et al. MRI features of neuronal intranuclear inclusion disease, combining visual and quantitative imaging investigations. J Neuroradiol. 2024;51(3):274–80.
Google Scholar
Okamura S, et al. A case of neuronal intranuclear inclusion disease with recurrent vomiting and without apparent DWI abnormality for the first seven years. Heliyon. 2020;6(8):e04675.
Google Scholar
Liu Y, et al. Clinical and mechanism advances of neuronal intranuclear inclusion disease. Front Aging Neurosci. 2022;14:934725.
Google Scholar
Liang H, et al. Clinical and pathological features in adult-onset NIID patients with cortical enhancement. J Neurol. 2020;267(11):3187–98.
Google Scholar
Shen Y, et al. Encephalitis-like episodes with cortical edema and enhancement in patients with neuronal intranuclear inclusion disease. Neurol Sci. 2024;45(9):4501–11.
Google Scholar
Jacobs AH, Tavitian B. Noninvasive molecular imaging of neuroinflammation. J Cereb Blood Flow Metab. 2012;32(7):1393–415.
Google Scholar
Corica F et al. PET imaging of Neuro-Inflammation with tracers targeting the translocator protein (TSPO), a systematic review: from bench to bedside. Diagnostics (Basel), 2023;13(6):1029.
Banati RB. Visualising microglial activation in vivo. Glia. 2002;40(2):206–17.
Google Scholar
Turner MR, et al. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11 C](R)-PK11195 positron emission tomography study. Neurobiol Dis. 2004;15(3):601–9.
Google Scholar
Tondo G, et al. (11) C-PK11195 PET-based molecular study of microglia activation in SOD1 amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2020;7(9):1513–23.
Google Scholar
Malpetti M, et al. Microglial activation in the frontal cortex predicts cognitive decline in frontotemporal dementia. Brain. 2023;146(8):3221–31.
Google Scholar
Zhong S, et al. Microglia contribute to polyG-dependent neurodegeneration in neuronal intranuclear inclusion disease. Acta Neuropathol. 2024;148(1):21.
Google Scholar
Hutton BF. The origins of SPECT and SPECT/CT. Eur J Nucl Med Mol Imaging. 2014;41(Suppl 1):S3-16.
Google Scholar
Benadiba M, et al. New molecular targets for PET and SPECT imaging in neurodegenerative diseases. Braz J Psychiatry. 2012;34(Suppl 2):S125–36.
Google Scholar
Kimachi T, et al. Reversible encephalopathy with focal brain edema in patients with neuronal intranuclear inclusion disease. Neurology and Clinical Neuroscience. 2017;5(6):198–200.
Google Scholar
Fujita K, et al. Neurologic attack and dynamic perfusion abnormality in neuronal intranuclear inclusion disease. Neurol Clin Pract. 2017;7(6):e39-42.
Google Scholar
Pan Y, et al. Expression of expanded GGC repeats within NOTCH2NLC causes cardiac dysfunction in mouse models. Cell Biosci. 2023;13(1):157.
Google Scholar
Heslegrave A, et al. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer’s disease. Mol Neurodegener. 2016;11:3.
Google Scholar
Craig-Schapiro R, et al. YKL-40: a novel prognostic fluid biomarker for preclinical alzheimer’s disease. Biol Psychiatry. 2010;68(10):903–12.
Google Scholar
Crols R, et al. Increased GFAP levels in CSF as a marker of organicity in patients with Alzheimer’s disease and other types of irreversible chronic organic brain syndrome. J Neurol. 1986;233(3):157–60.
Google Scholar
Andrés-Benito P, et al. Differential astrocyte and oligodendrocyte vulnerability in murine Creutzfeldt-Jakob disease. Prion. 2021;15(1):112–20.
Google Scholar
Mussbacher M, et al. NF-κB in monocytes and macrophages – an inflammatory master regulator in multitalented immune cells. Front Immunol. 2023;14:1134661.
Google Scholar
Biasizzo M, Kopitar-Jerala N. Interplay between NLRP3 inflammasome and autophagy. Front Immunol. 2020;11:591803.
Google Scholar
Deng Q, et al. Pseudomonas aeruginosa triggers macrophage autophagy to escape intracellular killing by activation of the NLRP3 inflammasome. Infect Immun. 2016;84(1):56–66.
Google Scholar
Geissmann F, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–61.
Google Scholar
Guilliams M, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14(8):571–8.
Google Scholar
Prinz M, Jung S, Priller J. Microglia Biology: One Century Evol Concepts Cell. 2019;179(2):292–311.
Google Scholar
Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18(4):225–42.
Google Scholar
Liddelow SA, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–7.
Google Scholar
Keren-Shaul H, et al. A unique microglia type associated with restricting development of alzheimer’s disease. Cell. 2017;169(7):1276–e129017.
Google Scholar
Mathys H, et al. Temporal tracking of microglia activation in neurodegeneration at single-cell resolution. Cell Rep. 2017;21(2):366–80.
Google Scholar
Zhou Y, et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat Med. 2020;26(1):131–42.
Google Scholar
Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44(3):439–49.
Google Scholar
Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514.
Google Scholar
Merad M, et al. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604.
Google Scholar
Sun R, Jiang H. Border-associated macrophages in the central nervous system. J Neuroinflammation. 2024;21(1):67.
Google Scholar
Goldmann T, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016;17(7):797–805.
Google Scholar
Van Hove H, et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci. 2019;22(6):1021–35.
Google Scholar
Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118(1):103–13.
Google Scholar
Faraco G, et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest. 2016;126(12):4674–89.
Google Scholar
Rua R, et al. Infection drives meningeal engraftment by inflammatory monocytes that impairs CNS immunity. Nat Immunol. 2019;20(4):407–19.
Google Scholar
Dani N, et al. A cellular and Spatial map of the choroid plexus across brain ventricles and ages. Cell. 2021;184(11):3056–e307421.
Google Scholar
Mrdjen D, et al. High-Dimensional Single-Cell mapping of central nervous system immune cells reveals distinct myeloid subsets in Health, Aging, and disease. Immunity. 2018;48(2):380–95. .e6.
Google Scholar
Park L, et al. Brain perivascular macrophages initiate the neurovascular dysfunction of Alzheimer Aβ peptides. Circ Res. 2017;121(3):258–69.
Google Scholar
Schläger C, et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature. 2016;530(7590):349–53.
Google Scholar
Herz J, et al. Role of neutrophils in exacerbation of brain injury after focal cerebral ischemia in hyperlipidemic mice. Stroke. 2015;46(10):2916–25.
Google Scholar
Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–60.
Google Scholar
Greenberg SM, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165–74.
Google Scholar
Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry. 2012;83(2):124–37.
Google Scholar
Liao YC, et al. NOTCH2NLC GGC repeat expansion in patients with vascular leukoencephalopathy. Stroke. 2023;54(5):1236–45.
Google Scholar
Bao L, et al. GGC repeat expansions in NOTCH2NLC cause uN2CpolyG cerebral amyloid angiopathy. Brain. 2025;148(2):467–79.
Google Scholar
Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J Cell Mol Med. 2009;13(9a):2911–25.
Google Scholar
Shan Y, et al. The glucagon-like peptide-1 receptor agonist reduces inflammation and blood-brain barrier breakdown in an astrocyte-dependent manner in experimental stroke. J Neuroinflammation. 2019;16(1):242.
Google Scholar
Orihara A, et al. Acute reversible encephalopathy with neuronal intranuclear inclusion disease diagnosed by a brain biopsy: inferring the mechanism of encephalopathy from radiological and histological findings. Intern Med. 2023;62(12):1821–5.
Google Scholar
Li YY, et al. Interactions between beta-Amyloid and pericytes in Alzheimer’s disease. Front Biosci (Landmark Ed). 2024;29(4):136.
Google Scholar
Huang X, Hussain B, Chang J. Peripheral inflammation and blood-brain barrier disruption: effects and mechanisms. CNS Neurosci Ther. 2021;27(1):36–47.
Google Scholar
Rahimifard M, et al. Targeting the TLR4 signaling pathway by polyphenols: a novel therapeutic strategy for neuroinflammation. Ageing Res Rev. 2017;36:11–9.
Google Scholar
Zhang M, et al. Blockage of VEGF function by bevacizumab alleviates early-stage cerebrovascular dysfunction and improves cognitive function in a mouse model of Alzheimer’s disease. Transl Neurodegener. 2024;13(1):1.
Google Scholar
Wild CP. Complementing the genome with an exposome: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847–50.
Google Scholar
Doroszkiewicz J et al. Common and trace metals in alzheimer’s and parkinson’s diseases. Int J Mol Sci, 2023;24(21):15721.
Gu Y, et al. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer’s disease. J Alzheimers Dis. 2010;22(2):483–92.
Google Scholar
Flanagan E, et al. Nutrition and the ageing brain: moving towards clinical applications. Ageing Res Rev. 2020;62:101079.
Google Scholar
Gelpi E, et al. Neuronal intranuclear (hyaline) inclusion disease and fragile X-associated tremor/ataxia syndrome: a morphological and molecular dilemma. Brain. 2017;140(8):e51.
Google Scholar
Tolar M et al. Neurotoxic soluble amyloid oligomers drive alzheimer’s pathogenesis and represent a clinically validated target for slowing disease progression. Int J Mol Sci, 2021;22(12):6355.
Habashi M, et al. Early diagnosis and treatment of alzheimer’s disease by targeting toxic soluble Aβ oligomers. Proc Natl Acad Sci U S A. 2022;119(49):e2210766119.
Google Scholar
Wang ZX, et al. The essential role of soluble Aβ oligomers in alzheimer’s disease. Mol Neurobiol. 2016;53(3):1905–24.
Google Scholar
Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99(3):1325–80.
Google Scholar
Su WJ, et al. Antidiabetic drug glyburide modulates depressive-like behavior comorbid with insulin resistance. J Neuroinflammation. 2017;14(1):210.
Google Scholar
Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10(10):717–25.
Google Scholar
Zhang J, et al. Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice. J Neuroinflammation. 2018;15(1):37.
Google Scholar
Nie R, et al. Porphyromonas gingivalis infection induces Amyloid-β accumulation in Monocytes/Macrophages. J Alzheimers Dis. 2019;72(2):479–94.
Google Scholar
Kemaladewi DU, et al. Correction of a splicing defect in a mouse model of congenital muscular dystrophy type 1A using a homology-directed-repair-independent mechanism. Nat Med. 2017;23(8):984–9.
Google Scholar
Sarkar S, et al. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded Huntingtin and related proteinopathies. Cell Death Differ. 2009;16(1):46–56.
Google Scholar
Grima JC, et al. Mutant Huntingtin disrupts the nuclear pore complex. Neuron. 2017;94(1):93–e1076.
Google Scholar
Radiomics may offer a superior means to assess spinal cord pathology compared with visual assessment of MRI in patients with
The findings may make it easier for clinicians to more precisely understand…
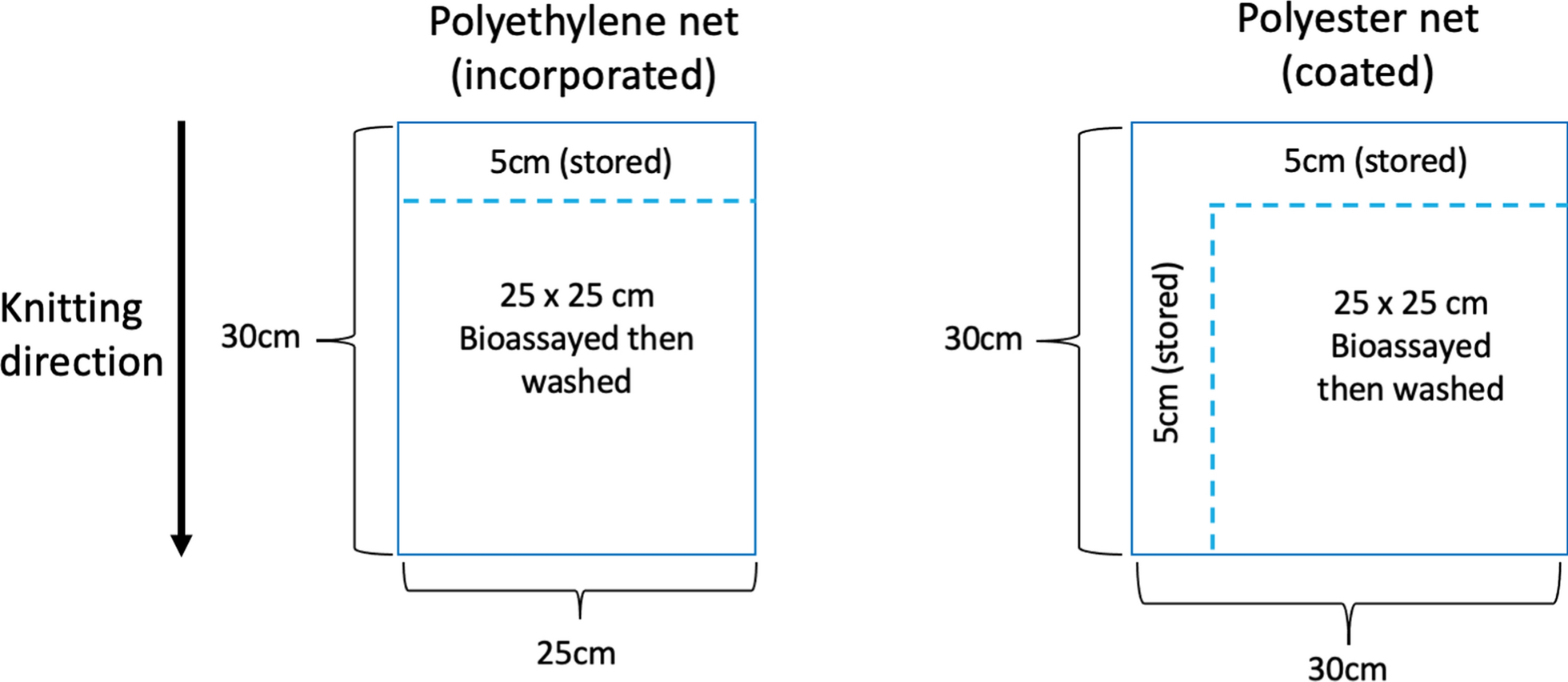
All tests were conducted at Biolytrics Vietnam between September 2018 and June 2020. Five experiments were performed. (1) validation of the GC-FID (Gas Chromatography with Flame Ionization Detection) compared to standard CIPAC HPLC (High…
Ahmed M, Zaghloul N, Zimmerman P, Casanova NG, Sun X, Reyes Hernon V, et al. Endothelial eNAMPT drives EndMT and preclinical PH: rescue by an eNAMPT-neutralizing mAb. Pulm Circ. 2021;11(4):20458940211059712.
Google Scholar
Chen J, Sysol JR, Singla S, Zhao S, Yamamura A, Valdez-Jasso D, et al. Nicotinamide phosphoribosyltransferase promotes pulmonary vascular remodeling and is a therapeutic target in pulmonary arterial hypertension. Circulation. 2017;135(16):1532–46.
Google Scholar
Sun X, Sun BL, Babicheva A, Vanderpool R, Oita RC, Casanova N, et al. Direct extracellular NAMPT involvement in pulmonary hypertension and vascular remodeling. Transcriptional regulation by SOX and HIF-2alpha. Am J Respir Cell Mol Biol. 2020;63(1):92–103.
Google Scholar
Gorelova A, Berman M, Al Ghouleh I. Endothelial-to-mesenchymal transition in pulmonary arterial hypertension. Antioxid Redox Signal. 2021;34(12):891–914.
Google Scholar
Pillai VB, Sundaresan NR, Kim G, Samant S, Moreno-Vinasco L, Garcia JG, et al. Nampt secreted from cardiomyocytes promotes development of cardiac hypertrophy and adverse ventricular remodeling. Am J Physiol Heart Circ Physiol. 2013;304(3):H415-26.
Google Scholar
Oita RC, Camp SM, Ma W, Ceco E, Harbeck M, Singleton P, et al. Novel mechanism for nicotinamide phosphoribosyltransferase Inhibition of TNF-alpha-mediated apoptosis in human lung endothelial cells. Am J Respir Cell Mol Biol. 2018;59(1):36–44.
Google Scholar
Casanova NG, Herazo-Maya J, Kempf CL, Sun BL, Song JH, Hernandez A et al. eNAMPT is a novel DAMP and therapeutic target in human and murine pulmonary fibrosis. . Am J Respir Cell Mol Biol. 2025.
Quijada H, Bermudez T, Kempf CL, Valera DG, Garcia AN, Camp SM et al. Endothelial eNAMPT amplifies preclinical acute lung injury: efficacy of an eNAMPT-Neutralising mAb. Eur Respir J. 2021;57(5):2002536.
Bao C, Liang S, Han Y, Yang Z, Liu S, Sun Y, et al. The novel lysosomal autophagy inhibitor (ROC-325) ameliorates experimental pulmonary hypertension. Hypertension. 2023;80(1):70–83.
Google Scholar
Tang C, Luo Y, Li S, Huang B, Xu S, Li L. Characteristics of inflammation process in monocrotaline-induced pulmonary arterial hypertension in rats. Biomed Pharmacother. 2021;133:111081.
Google Scholar
Moreno-Vinasco L, Gomberg-Maitland M, Maitland ML, Desai AA, Singleton PA, Sammani S, et al. Genomic assessment of a multikinase inhibitor, sorafenib, in a rodent model of pulmonary hypertension. Physiol Genomics. 2008;33(2):278–91.
Google Scholar
Tang H, Babicheva A, McDermott KM, Gu Y, Ayon RJ, Song S, et al. Endothelial HIF-2alpha contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition. Am J Physiol Lung Cell Mol Physiol. 2018;314(2):L256–75.
Google Scholar
Guo M, Morley MP, Jiang C, Wu Y, Li G, Du Y, et al. Guided construction of single cell reference for human and mouse lung. Nat Commun. 2023;14(1):4566.
Google Scholar
Tsukui T, Sun KH, Wetter JB, Wilson-Kanamori JR, Hazelwood LA, Henderson NC, et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat Commun. 2020;11(1):1920.
Google Scholar
Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, stringtie and ballgown. Nat Protoc. 2016;11(9):1650–67.
Google Scholar
Miller SA, Policastro RA, Sriramkumar S, Lai T, Huntington TD, Ladaika CA, et al. LSD1 and aberrant DNA methylation mediate persistence of enteroendocrine progenitors that support BRAF-mutant colorectal cancer. Cancer Res. 2021;81(14):3791–805.
Google Scholar
Gillich A, Zhang F, Farmer CG, Travaglini KJ, Tan SY, Gu M, et al. Capillary cell-type specialization in the alveolus. Nature. 2020;586(7831):785–9.
Google Scholar
Liu B, Yi D, Xia X, Ramirez K, Zhao H, Cao Y, et al. General capillary endothelial cells undergo reprogramming into arterial endothelial cells in pulmonary hypertension through HIF-2alpha/Notch4 pathway. Circulation. 2024;150(5):414–7.
Google Scholar
Man S, Sanchez Duffhues G, Ten Dijke P, Baker D. The therapeutic potential of targeting the endothelial-to-mesenchymal transition. Angiogenesis. 2019;22(1):3–13.
Google Scholar
Hong J, Arneson D, Umar S, Ruffenach G, Cunningham CM, Ahn IS, et al. Single-cell study of two rat models of pulmonary arterial hypertension reveals connections to human pathobiology and drug repositioning. Am J Respir Crit Care Med. 2021;203(8):1006–22.
Google Scholar
Tarhan L, Bistline J, Chang J, Galloway B, Hanna E, Weitz E. Single cell portal: an interactive home for single-cell genomics data. bioRxiv. 2023. https://doi.org/10.1101/2023.07.13.548886.
Google Scholar
Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115(1):165–75.
Google Scholar
Ahmed M, Casanova NG, Zaghloul N, Gupta A, Rodriguez M, Robbins IR, et al. The eNAMPT/TLR4 inflammatory cascade drives the severity of intra-amniotic inflammation in pregnancy and predicts infant outcomes. Front Physiol. 2023;14:1129413.
Google Scholar
Bime C, Casanova NG, Nikolich-Zugich J, Knox KS, Camp SM, Garcia JGN. Strategies to dampen COVID-19-mediated lung and systemic inflammation and vascular injury. Transl Res. 2021;232:374–8. https://doi.org/10.1016/j.trsl.2020.12.008.
Gao L, Ramirez FJ, Cabrera JTO, Varghese MV, Watanabe M, Tsuji-Hosokawa A, et al. eNAMPT is a novel therapeutic target for mitigation of coronary microvascular disease in type 2 diabetes. Diabetologia. 2024;67(9):1998–2011.
Google Scholar
Sun BL, Sun X, Casanova N, Garcia AN, Oita R, Algotar AM, et al. Role of secreted extracellular nicotinamide phosphoribosyltransferase (eNAMPT) in prostate cancer progression: novel biomarker and therapeutic target. EBioMedicine. 2020;61:103059.
Google Scholar
Sun BL, Sun X, Kempf CL, Song JH, Casanova NG, Camp SM, et al. Involvement of eNAMPT/TLR4 inflammatory signaling in progression of non-alcoholic fatty liver disease, steatohepatitis, and fibrosis. FASEB J. 2023;37(3):e22825.
Google Scholar
Tumurkhuu G, Casanova NG, Kempf CL, Ercan Laguna D, Camp SM, Dagvadorj J, et al. eNAMPT/TLR4 inflammatory cascade activation is a key contributor to SLE lung vasculitis and alveolar hemorrhage. J Transl Autoimmun. 2023;6:100181.
Google Scholar
Ye SQ, Simon BA, Maloney JP, Zambelli-Weiner A, Gao L, Grant A, et al. Pre-B-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am J Respir Crit Care Med. 2005;171(4):361–70.
Google Scholar
Alvandi Z, Bischoff J. Endothelial-mesenchymal transition in cardiovascular disease. Arterioscler Thromb Vasc Biol. 2021;41(9):2357–69.
Google Scholar
Huang J, Pu Y, Zhang H, Xie L, He L, Zhang CL, et al. KLF2 mediates the suppressive effect of laminar flow on vascular calcification by inhibiting endothelial BMP/SMAD1/5 signaling. Circ Res. 2021;129(4):e87-100.
Google Scholar
Kang K, Xiang J, Zhang X, Xie Y, Zhou M, Zeng L, et al. N6-methyladenosine modification of KLF2 may contribute to endothelial-to-mesenchymal transition in pulmonary hypertension. Cell Mol Biol Lett. 2024;29(1):69.
Google Scholar
Zhang-Sun ZY, Xu XZ, Escames G, Lei WR, Zhao L, Zhou YZ, et al. Targeting NR1D1 in organ injury: challenges and prospects. Mil Med Res. 2023;10(1):62.
Google Scholar
Chae WJ, Bothwell ALM. Canonical and non-canonical Wnt signaling in immune cells. Trends Immunol. 2018;39(10):830–47.
Google Scholar
Sun L, Xing J, Zhou X, Song X, Gao S. Wnt/beta-catenin signalling, epithelial-mesenchymal transition and crosslink signalling in colorectal cancer cells. Biomed Pharmacother. 2024;175:116685.
Google Scholar
Tang H, Wu K, Wang J, Vinjamuri S, Gu Y, Song S, et al. Pathogenic role of mTORC1 and mTORC2 in pulmonary hypertension. JACC Basic Transl Sci. 2018;3(6):744–62.
Google Scholar
Lowe M, Lage J, Paatela E, Munson D, Hostager R, Yuan C, et al. Cry2 is critical for circadian regulation of myogenic differentiation by Bclaf1-mediated mRNA stabilization of Cyclin D1 and Tmem176b. Cell Rep. 2018;22(8):2118–32.
Google Scholar
Bryant AJ, Ebrahimi E, Nguyen A, Wolff CA, Gumz ML, Liu AC, Esser KA. A wrinkle in time: circadian biology in pulmonary vascular health and disease. Am J Physiol Lung Cell Mol Physiol. 2022;322(1):L84–101.
Google Scholar
Lopez-Moncada F, Torres MJ, Lavanderos B, Cerda O, Castellon EA, Contreras HR. SPARC induces E-Cadherin repression and enhances cell migration through integrin alphavbeta3 and the transcription factor ZEB1 in prostate cancer cells. Int J Mol Sci. 2022;23(11):5874. https://doi.org/10.3390/ijms23115874.
Dean A, Gregorc T, Docherty CK, Harvey KY, Nilsen M, Morrell NW, et al. Role of the aryl hydrocarbon receptor in Sugen 5416-induced experimental pulmonary hypertension. Am J Respir Cell Mol Biol. 2018;58(3):320–30.
Google Scholar
Liang S, Guo H, Ma K, Li X, Wu D, Wang Y, et al. A PLCB1-PI3K-AKT signaling axis activates EMT to promote cholangiocarcinoma progression. Cancer Res. 2021;81(23):5889–903.
Google Scholar
Karoor V, Fini MA, Loomis Z, Sullivan T, Hersh LB, Gerasimovskaya E, et al. Sustained activation of Rho GTPases promotes a synthetic pulmonary artery smooth muscle cell phenotype in Neprilysin null mice. Arterioscler Thromb Vasc Biol. 2018;38(1):154–63.
Google Scholar
Alexander MR, Murgai M, Moehle CW, Owens GK. Interleukin-1beta modulates smooth muscle cell phenotype to a distinct inflammatory state relative to PDGF-DD via NF-kappaB-dependent mechanisms. Physiol Genomics. 2012;44(7):417–29.
Google Scholar
Cho DI, Ahn MJ, Cho HH, Cho M, Jun JH, Kang BG, et al. Angptl4 stabilizes atherosclerotic plaques and modulates the phenotypic transition of vascular smooth muscle cells through KLF4 downregulation. Exp Mol Med. 2023;55(2):426–42.
Google Scholar
Cheung AK, Ko JM, Lung HL, Chan KW, Stanbridge EJ, Zabarovsky E, et al. Cysteine-rich intestinal protein 2 (CRIP2) acts as a repressor of NF-kappaB-mediated proangiogenic cytokine transcription to suppress tumorigenesis and angiogenesis. Proc Natl Acad Sci U S A. 2011;108(20):8390–5.
Google Scholar
Yang L, Zhou F, Zheng D, Wang D, Li X, Zhao C, Huang X. FGF/FGFR signaling: from lung development to respiratory diseases. Cytokine Growth Factor Rev. 2021;62:94–104.
Google Scholar
Wen Z, Jiang L, Yu F, Xu X, Chen M, Li C, et al. ScrNA-seq reveals NAMPT-mediated macrophage polarization shapes smooth muscle cell plasticity in pulmonary arterial hypertension. Interdiscip Med. 2024;2(4):e20240016.
Google Scholar
Streef TJ, Groeneveld EJ, van Herwaarden T, Hjortnaes J, Goumans MJ, Smits AM. Single-cell analysis of human fetal epicardium reveals its cellular composition and identifies CRIP1 as a modulator of EMT. Stem Cell Reports. 2023;18(7):1421–35.
Google Scholar
Ahmed MN, Zhang Y, Codipilly C, Zaghloul N, Patel D, Wolin M, Miller EJ. Extracellular superoxide dismutase overexpression can reverse the course of hypoxia-induced pulmonary hypertension. Mol Med. 2012;18:38–46.
Google Scholar
Tarazona S, Furio-Tari P, Turra D, Pietro AD, Nueda MJ, Ferrer A, et al. Data quality aware analysis of differential expression in RNA-seq with NOIseq r/bioc package. Nucleic Acids Res. 2015;43(21):e140.
Google Scholar
Zawia A, Arnold ND, West L, Pickworth JA, Turton H, Iremonger J, et al. Altered macrophage polarization induces experimental pulmonary hypertension and is observed in patients with pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2021;41(1):430–45.
Google Scholar
Qiu H, He Y, Ouyang F, Jiang P, Guo S, Guo Y. The role of regulatory T cells in pulmonary arterial hypertension. J Am Heart Assoc. 2019;8(23):e014201.
Google Scholar
Rodriguez M, Xu H, Hernandez A, Ingraham J, Canizales Galaviz J, Teran Arce F et al. NEDD4 E3 ligase-catalyzed NAMPT ubiquitination and autophagy are essential for pyroptosis-independent NAMPT secretion in human monocytes. Cell Commun Signal. 2025;23:157 https://doi.org/10.1186/s12964-025-02164-5.
Audrito V, Serra S, Brusa D, Mazzola F, Arruga F, Vaisitti T, et al. Extracellular nicotinamide phosphoribosyltransferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia. Blood. 2015;125(1):111–23.
Google Scholar
Wang Z, Li W, Chen S, Tang XX. Role of ADAM and ADAMTS proteases in pathological tissue remodeling. Cell Death Discov. 2023;9(1):447.
Google Scholar
Hudalla H, Michael Z, Christodoulou N, Willis GR, Fernandez-Gonzalez A, Filatava EJ, et al. Carbonic anhydrase inhibition ameliorates inflammation and experimental pulmonary hypertension. Am J Respir Cell Mol Biol. 2019;61(4):512–24.
Google Scholar
Zhu Y, Dun H, Ye L, Terada Y, Shriver LP, Patti GJ et al. Targeting fatty acid beta-oxidation impairs monocyte differentiation and prolongs heart allograft survival. JCI Insight. 2022;7(7):e151596. https://doi.org/10.1172/jci.insight.151596.
Tyrrell C, De Cecco M, Reynolds NL, Kilanowski F, Campopiano D, Barran P, et al. Isoleucine/leucine2 is essential for chemoattractant activity of beta-defensin Defb14 through chemokine receptor 6. Mol Immunol. 2010;47(6):1378–82.
Google Scholar
Cai X, Deng J, Ming Q, Cai H, Chen Z. Chemokine-like factor 1: a promising therapeutic target in human diseases. Exp Biol Med (Maywood). 2020;245(16):1518–28.
Google Scholar
Liao Y, Deng C, Wang X. VSIG4 ameliorates intestinal inflammation through inhibiting macrophages NLRP3 inflammasome and pyroptosis. Tissue Cell. 2024;86:102285.
Google Scholar
Li R, Chen B, Kubota A, Hanna A, Humeres C, Hernandez SC, et al. Protective effects of macrophage-specific integrin alpha5 in myocardial infarction are associated with accentuated angiogenesis. Nat Commun. 2023;14(1):7555.
Google Scholar
Jairath V, Acosta Felquer ML, Cho RJ. IL-23 inhibition for chronic inflammatory disease. Lancet. 2024;404(10463):1679–92.
Google Scholar
Tang C, Chen S, Qian H, Huang W. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology. 2012;135(2):112–24.
Google Scholar
Garcia AN, Casanova NG, Valera DG, Sun X, Song JH, Kempf CL, et al. Involvement of eNAMPT/TLR4 signaling in murine radiation pneumonitis: protection by eNAMPT neutralization. Transl Res. 2022;239:44–57.
Google Scholar
Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54(1 Suppl):S10–9.
Google Scholar
Pullamsetti SS, Savai R, Janssen W, Dahal BK, Seeger W, Grimminger F, et al. Inflammation, immunological reaction and role of infection in pulmonary hypertension. Clin Microbiol Infect. 2011;17(1):7–14.
Google Scholar
Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122(9):920–7.
Google Scholar
Wang J, Tian XT, Peng Z, Li WQ, Cao YY, Li Y, Li XH. HMGB1/TLR4 promotes hypoxic pulmonary hypertension via suppressing BMPR2 signaling. Vascul Pharmacol. 2019;117:35–44.
Google Scholar
Xiao G, Zhuang W, Wang T, Lian G, Luo L, Ye C, et al. Transcriptomic analysis identifies toll-like and nod-like pathways and necroptosis in pulmonary arterial hypertension. J Cell Mol Med. 2020;24(19):11409–21.
Google Scholar
Young KC, Hussein SM, Dadiz R, deMello D, Devia C, Hehre D, et al. Toll-like receptor 4-deficient mice are resistant to chronic hypoxia-induced pulmonary hypertension. Exp Lung Res. 2010;36(2):111–9.
Google Scholar
Dai Z, Li M, Wharton J, Zhu MM, Zhao YY. Prolyl-4 hydroxylase 2 (PHD2) deficiency in endothelial cells and hematopoietic cells induces obliterative vascular remodeling and severe pulmonary arterial hypertension in mice and humans through hypoxia-inducible factor-2alpha. Circulation. 2016;133(24):2447–58.
Google Scholar

Paris, France, 29 October 2025 – His Highness the Aga Khan was received by President Emmanuel Macron at the Élysée Palace yesterday. The meeting follows the signing of an agreement with the government of France in July 2025 to work together…

Danny FullbrookBedfordshire, Hertfordshire and Buckinghamshire
 Getty Images
Getty ImagesA company that ran an immersive horror attraction has gone bust…

When Phia‘s founders Phoebe Gates and Sophia Kianni decided to build an AI startup, they targeted an area they understood well: online shopping.
The founders, who met at Stanford when they were randomly paired up as roommates, understood…