At the ESMO Congress 2025 in Berlin, Dr. Joseph Tintelnot presented the interim analysis of the phase II PHERFLOT/IKF-053 trial (AIO STO 0321), an open-label, multicenter study evaluating whether adding pembrolizumab (anti-PD-1) and trastuzumab (anti-HER2) to perioperative FLOT chemotherapy (5-FU, leucovorin, oxaliplatin, docetaxel) could improve outcomes for patients with HER2-positive localized esophagogastric adenocarcinoma (EGA). Conducted by the AIO Study Group in Germany, this investigation explores the integration of immune- and targeted-therapy principles into a curative-intent treatment paradigm.
Background
For advanced or metastatic HER2-positive EGA, the combination of fluoropyrimidine-platinum chemotherapy with pembrolizumab and trastuzumab is already a standard of care, based on the positive results of the KEYNOTE-811 study. In localized disease, however, perioperative FLOT remains the preferred backbone due to its proven survival advantage over older ECF-based regimens. The PHERFLOT trial was designed to determine whether adding dual HER2 and PD-1 blockade could deepen pathological responses and potentially improve long-term survival without compromising surgical feasibility.
Study Design
PHERFLOT (NCT05504720) is a randomized, open-label, exploratory phase II study enrolling patients with resectable, HER2-positive localized EGA. The co-primary endpoints are 2-year disease-free survival and pathological complete response (pCR). Secondary objectives include safety, progression-free survival, objective response rate, and translational biomarker analyses, such as HER2 expression and PD-L1 combined positive score (CPS).
Patients received standard FLOT chemotherapy combined with pembrolizumab and trastuzumab in both the neoadjuvant and adjuvant settings, followed by curative-intent resection. The current report summarizes baseline characteristics, pathological outcomes, safety, and preliminary translational findings.
Results
Between March 2023 and May 2024, 31 patients were enrolled across multiple German centers. The median age was 65 years, and most had an ECOG performance status of 0 or 1. The majority of tumors arose at the gastroesophageal junction (77%), while a smaller proportion involved the stomach proper. HER2 overexpression was confirmed by immunohistochemistry, with 80.6% scoring 3+. PD-L1 CPS was ≥1 in 85% and ≥10 in half of evaluated cases, reflecting a generally inflamed tumor phenotype.
Thirty patients proceeded to R0 resection, and 70% experienced no postoperative complications, underscoring the feasibility of combining immune and targeted agents with intensive triplet chemotherapy. The pCR rate reached 48.4% (15 of 31), meeting the co-primary endpoint and far exceeding historical FLOT-alone benchmarks of roughly 15–20%. When subtotal responses were included, two-thirds of patients (67.8%) achieved major regression.
Twenty-one of 31 patients continued beyond perioperative therapy, corresponding to a feasibility rate of 67.7%.
The safety profile was consistent with expectations, except for an increased incidence of Grade 3 diarrhea (38.7%) and a higher re-operation rate (26.7% vs. 10% reported in FLOT4). The most frequent treatment-related events included diarrhea, weight loss, and hematologic toxicities such as neutropenia and anemia. The median hospitalization time was 14 days (vs 15 days in FLOT4), and no 30-day postoperative mortality occurred, confirming the acceptable perioperative safety of the regimen.
Translational Findings
Initial biomarker analyses suggest that PD-L1 expression may correlate with the depth of response, though patient numbers remain small. Ongoing translational work will explore the interaction between HER2 signaling, immune activation, and tumor regression, including immune-cell infiltration and circulating tumor DNA (ctDNA) kinetics. These data may ultimately clarify which subgroups derive the greatest benefit from the triplet combination.
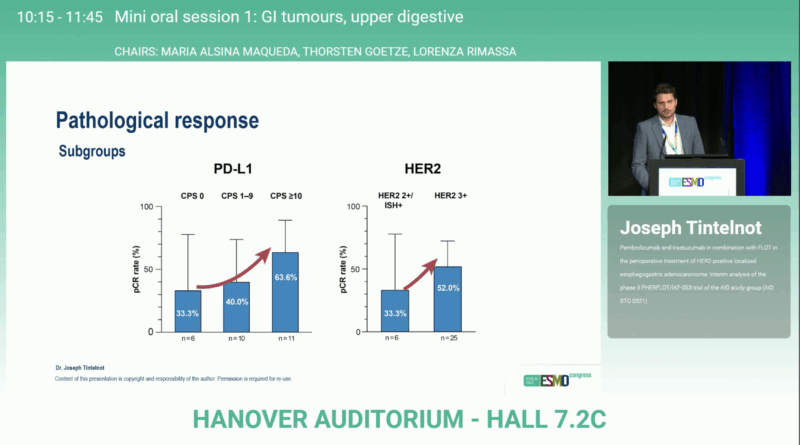
Notably, higher pathological complete response rates were observed in patients with PD-L1 CPS ≥10 (63.6%) and in those with HER2 3+ expression (52%), indicating enhanced treatment sensitivity in these biomarker-enriched subgroups.
You can read the full abstract here.
Conclusions
The interim findings from PHERFLOT/IKF-053 demonstrate that combining pembrolizumab and trastuzumab with perioperative FLOT is feasible, tolerable, and highly active in localized HER2-positive esophagogastric adenocarcinoma. With nearly half of patients achieving complete pathological response and two-thirds achieving major regression, the regimen shows clear biological synergy and potential superiority over current standards.
The long-term results—particularly the 2-year disease-free survival data—will determine whether this intensified perioperative strategy can establish a new benchmark for this molecularly defined subgroup. If confirmed, PHERFLOT could represent a major step toward incorporating immunotherapy and HER2-targeted treatment into curative pathways for upper-GI cancers.

You Can Also Read MATTERHORN Trial at ESMO 2025: Durvalumab Plus FLOT in Resectable Gastric and GEJ Adenocarcinoma by OncoDaily




