INEOS Grenadiers are proud to announce the signing of exciting French rider Kévin Vauquelin, who will join the team on a three-year deal from 2026.
At just 24 years old, Vauquelin’s performances have continued to evolve including…

INEOS Grenadiers are proud to announce the signing of exciting French rider Kévin Vauquelin, who will join the team on a three-year deal from 2026.
At just 24 years old, Vauquelin’s performances have continued to evolve including…
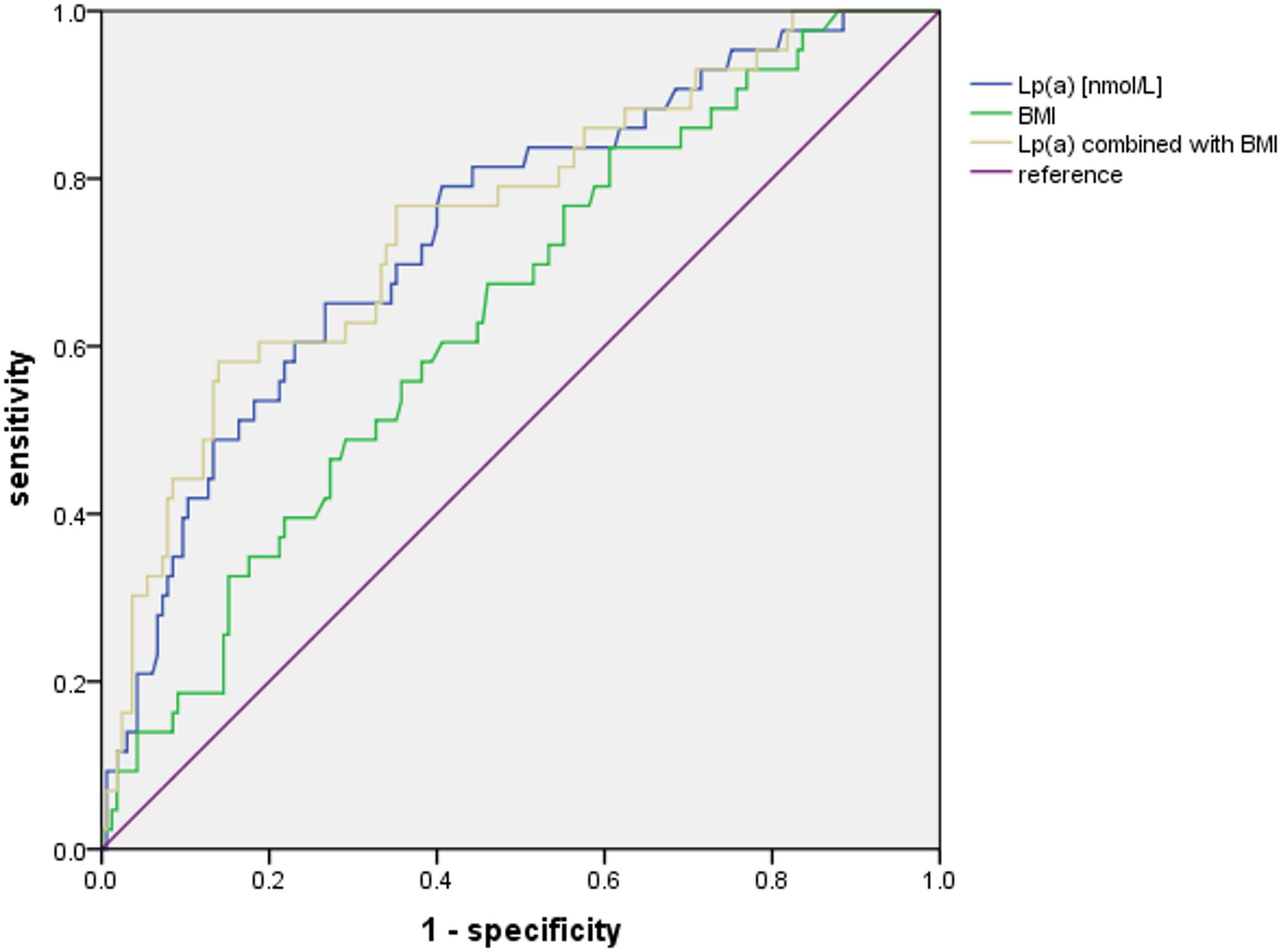
Our study demonstrated the predictive value of Lp(a) for new-onset AVC in patients with CAD, and further explored and evaluated other risk factors of AVC.
It is known that higher Lp(a) level is associated with CAD and reduce Lp(a) is undoubtedly…
ISLAMABAD, Oct. 10 (Xinhua) — Eight Pakistani soldiers were killed and five others injured when militants opened fire on an army post and a security patrol in Pakistan’s northwest Khyber Pakhtunkhwa province early Friday…

The Pakistani Army on Friday said that its security forces…

Strategic partnership aims to unlock new opportunities in digital construction by combining global software leadership with cutting-edge wearable technologies and deep regional expertise
Riyadh, Saudi Arabia – 9 October 2025 – The Nemetschek Group, one of the world’s leading software providers for the Architecture, Engineering, Construction and Operations (AEC/O) industry, has partnered with WakeCap Technologies, the region’s leading construction technology company, specializing in sensor-powered, real-time project control systems. This landmark agreement sets the stage for a strategic collaboration aimed at accelerating digital transformation across the Gulf Cooperation Council’s (GCC) construction ecosystem.
The partnership leverages the strengths of both companies: Nemetschek Group’s extensive portfolio of industry-leading software solutions and WakeCap’s cutting-edge sensor-powered project-controls platform, which delivers real-time workforce visibility and site-wide intelligence. Currently deployed across $100B+ in giga-projects with the region’s most extensive workforce database tracking over 150 million worker hours, WakeCap has established itself as the only platform operating at this scale in the Middle East. The system has delivered transformative results by driving behavioral change on construction sites, achieving 90%+ reduction in safety violation observations, 25%+ gains in productivity, and 70%+ faster incident response. WakeCap safety solutions are now mandated across Aramco, Neom, and Qiddiya.
The agreement outlines a comprehensive framework for both companies to jointly pursue business opportunities and initiatives within the GCC. WakeCap’s deep regional expertise and established relationships with the Middle East’s largest owners and contractors will accelerate Nemetschek Arabia and its affiliates’ market entry and expansion through localized expertise and access to an established network of industry partners. The collaboration encompasses the development of joint go-to-market strategies aimed at stakeholders in construction and real estate, as well as the potential integration of WakeCap’s technologies with Nemetschek’s leading software platforms – including Bluebeam, dTwin and GoCanvas.
Furthermore, the two organizations intend to collaborate on knowledge exchange and co-create thought leadership content, such as whitepapers and digital construction initiatives, while jointly participating in regional industry events. The partnership will also evaluate new synergies within the infrastructure and natural resource sectors, where both parties recognize increasing demand for intelligent, data-driven project delivery solutions.
Commenting on the partnership, Marc Nezet, Group Chief Strategy Officer and Chief Division Officer of the Nemetschek Group, said: “WakeCap is one of the most innovative frontrunners in the global construction tech landscape. Their ability to deliver real-time visibility and actionable insights at scale is reshaping how major infrastructure projects are built and managed. By combining Nemetschek’s world-class software capabilities with WakeCap’s cutting-edge solutions and regional strength, we are excited to unlock transformative outcomes for the GCC’s construction sector.”
Muayad Simbawa, Managing Director of Nemetschek Arabia, emphasized the significance of the agreement in supporting the region’s digital ambitions. “This collaboration marks a significant milestone in our mission to accelerate digital innovation in construction across the Gulf region. Together with WakeCap, we aim to deliver more connected, intelligent, and efficient project delivery models that align with the region’s national development agendas and sustainability goals.”
“This partnership validates our vision of transforming construction through real-time intelligence”, stated Dr. Hassan Albalawi, CEO and Founder of WakeCap. “We’ve proven our technology can fundamentally change how giga-projects are delivered, reducing risk, and driving efficiency. Now, by combining our field-proven platform with Nemetschek’s global reach and software ecosystem, we’re not just expanding our market, we’re defining the future of construction technology. Our joint solutions will become the global benchmark for intelligent project delivery, starting here in the GCC where the world’s most ambitious projects are already relying on WakeCap.
This agreement reinforces the Nemetschek Group’s long-term commitment to advancing digital innovation across global construction and infrastructure markets. With a portfolio of leading brands and platforms that span the entire AEC/O lifecycle, Nemetschek continues to deliver open, interoperable, and sustainable solutions that empower the world’s architects, engineers, contractors, and facility managers to build better.
The GCC construction market, valued at over $120 billion annually with several trillion-dollar national transformation programs underway, represents the ideal proving ground for these advanced technologies.
About WakeCap
WakeCap is the sensor-powered project controls platform that has become the technology partner for the Middle East’s most complex construction projects. Since pioneering construction wearables in 2017, WakeCap’s platform is currently deployed across $100B+ of active giga-projects, with over 150M work hours tracked.
WakeCap has delivered transformative results by driving behavioral change on construction sites, achieving 90%+ reduction in safety violation observations, 25%+ gains in productivity, and 70%+ faster incident response. The platform’s unique ability to operate without GPS, WiFi, or cabling through it’s self-forming mesh network has made it indispensable for high-risk and high-stakes projects. Trusted by industry giants including Aramco, Neom, Qiddiya, and the region’s top contractors, WakeCap is building the foundation for a smarter, safer, and more transparent construction industry.
ISLAMABAD, Oct. 10 (Xinhua) — Eight Pakistani soldiers were killed and five others injured when militants opened fire on an army post and a security patrol in Pakistan’s northwest Khyber Pakhtunkhwa province early Friday morning, security…

Final competition will take place in Milan in late October, 2025
LONDON – October 10, 2025 – Samsung Electronics Co., Ltd. announced today the 10 finalists of its inaugural European Samsung Solve for Tomorrow[1] Competition, which will take place in Milan, Italy, in late October.
The finalist ideas were selected by a panel of judges who evaluated candidates based on creativity (Idea), quality (Implementation) and impact (Scalability) — across different categories. These included Sustainability, Health, and “Social Change through Sport & Technology”. This last one is one of this year’s Global Themes after being voted on by the public in the Together for Tomorrow, Enabling People community. The newly launched theme reflects a shared commitment to inspire young people to become changemakers by combining the unifying power of sport with cutting-edge innovation.
“The quality and innovation demonstrated by the entries from across Europe in our Solve For Tomorrow 2025 program was stunning,” said Benjamin Braun, Chief Marketing Officer of Samsung Electronics Europe. “The 10 finalists should be very proud of this achievement, and we are looking forward to welcoming them to Milan”.
The 5 winner teams of the competition will have the opportunity to travel to Samsung’s HQ in Korea in 2026 for a guided visit that will hopefully inspire their innovative minds.
The 10 finalist ideas and their representatives[2] are:
|
Alicia & Gabriel SkillFIT Germany
|
SkillFIT is an AI-powered platform focuses on individual progress of physical education in schools rather than grades, supporting teachers in promoting movement with a focus on self-development and motivation. |
| Raye & Sarah
CuraStep UK
|
CuraStep, a smart IoT biometric shoe, empowers diabetic patients and individuals with foot ulcers to stay active and help them prevent complications by safeguarding against foot wounds. It offers potential benefits to a diverse audience with diabetic neuropathy, including professional athletes. |
| Filip & Juliusz
ExerTherapy Poland
|
ExerTherapy is a project supporting the well-being of students at school. A stationary bike for exercising between classes promotes youth activity and enables the release of emotions through pedaling, which also generates electrical energy. |
| Casciaro & Francesco
Kroove Italy
|
The Kroove mobile app connects young Italians through shared sports interests and local facilities, fostering inclusive communities and healthier lifestyles by bridging the gap between underutilized resources and the need for real-world social engagement. |
| Evan & Simon
Liova France
|
Liova is an eco-designed power bank using components recovered from old electronic devices such as unused phones. It’s an innovative solution that promotes a circular economy, as well as a smart and sustainable approach to modern energy needs. By giving a second life to these components, the students created added value and demonstrated key skills expected in a business environment. |
| Deimante & Rapolas,
Minova Lithuania
|
By leveraging AI to analyze historical sales and external factors like weather and events, the Minova solution optimizes restaurant inventory management, reducing food waste and saving costs while contributing to climate change mitigation |
| Petra & Zora
My Hormone Hungary
|
My Hormone provides comprehensive educational resources on female hormonal balance, addressing gaps in public health education and gaining support from professional organizations to empower long-term understanding and management of complex conditions like Polycystic Ovary Syndrome (PCOS). |
| Robert-Gabriel and Ilie-Ion,
Optimum Romania
|
The Optimum smart sneaker revolutionizes sports and fitness by combining sustainability, durability, comfort and advanced health monitoring, allowing users to track pulse and blood oxygen levels seamlessly while ensuring protection against water, dust and injury during any activity.
|
| Afroditi,
Supervision Greece
|
The “Braille Voice” smart voice system, awarded for its tech innovation and social impact, helps visually impaired shoppers by providing detailed product information — such as brand, price and usage instructions — through seamless voice assistance at supermarket shelves. |
| Jakub and Jakub,
VisionNex Czech Republic
|
These affordable smart glasses use AI to translate visual scenes into spoken descriptions. This empowers visually impaired individuals with assistance in daily orientation and sports participation, offering a life-enhancing solution at a fraction of the cost of existing devices. |

The Samsung Solve for Tomorrow program is active in 20 countries[3] across Europe, with each nation selecting their best ideas to solve community-level issues with technology.
Visit these websites for more information on Solve for Tomorrow:
https://csr.samsung.com/en/program/samsung-solve-for-tomorrow
https://news.samsung.com/global/samsung-solve-for-tomorrow-introduces-global-themes-to-unite-student-innovators-worldwide
https://news.samsung.com/global/samsung-solve-for-tomorrow-meets-the-olympic-spirit-dreaming-of-a-new-future-through-technology-and-sport-with-the-ioc
https://www.olympics.com/ioc/news/together-for-tomorrow-enabling-people-ioc-and-samsung-launch-new-digital-community-to-engage-young-olympic-fans-and-drive-positive-change
Samsung CSR
[1] Samsung Solve for Tomorrow was launched in 2010 and is a global problem-solving platform where youth around the world use STEM (science, technology, engineering and math) skills to solve real-world issues in their communities to build a better future together. Over the past 15 years, almost 2.8 million students in over 68 countries have participated in the program. Launched in the United States in 2010, it began its European expansion in 2019, initially launching in Germany, Switzerland, and Italy. As of 2025, the program is now active in a total of 20 European countries, with approximately 38,000 participants in 2024.
[2] For those teams comprised of more than 2 people, they will be represented by the finalists featured in this press release.
[3] Austria, Belgium, Bulgaria, Czech Republic, Estonia, Finland, France, Germany, Greece, Hungary, Italy, Latvia, Lithuania, Poland, Romania, San Marino, Slovakia, Switzerland, The Netherlands, U.K.
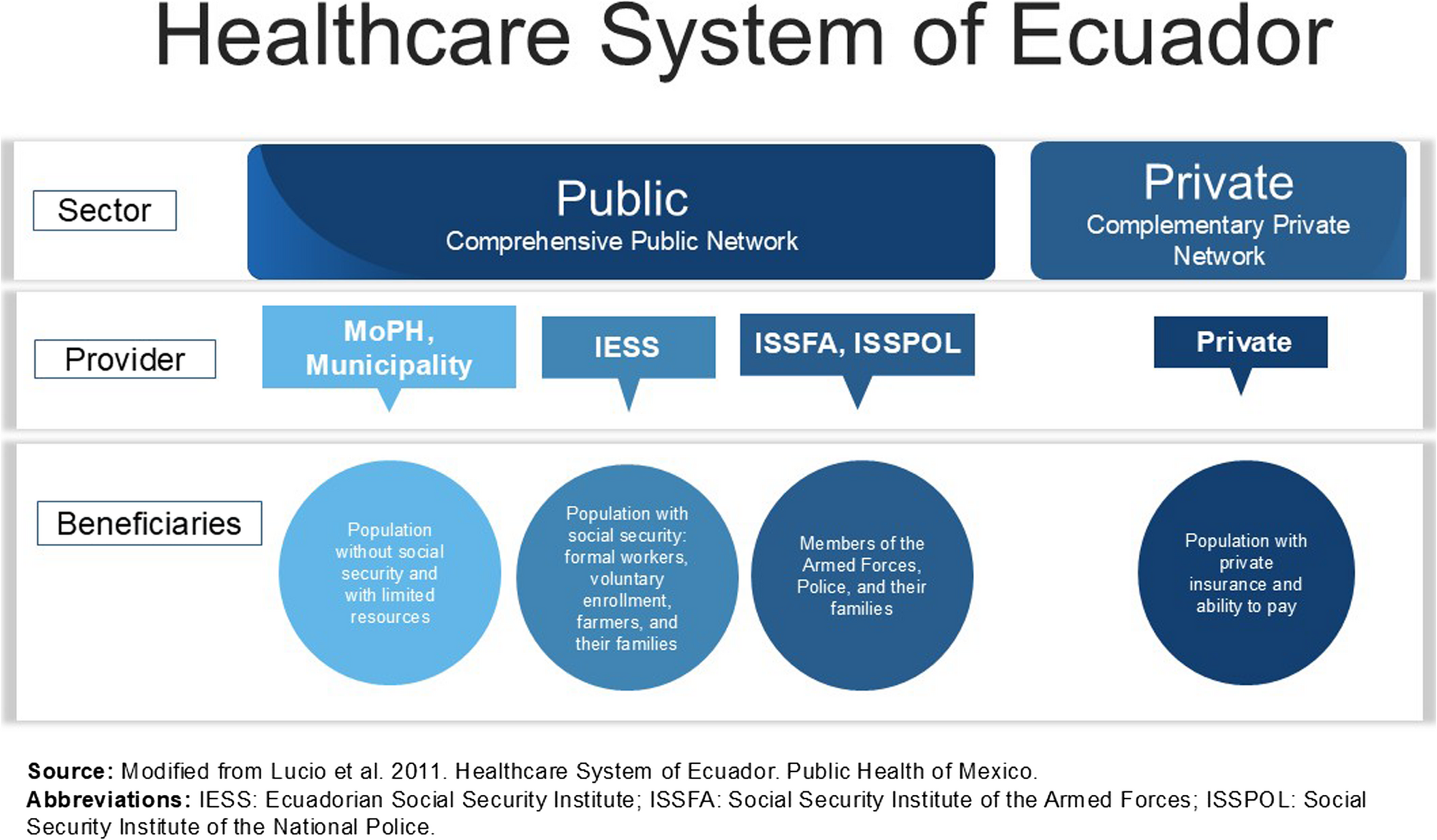
This study explored transforming and institutionalising a new community-based PHC model in the Metropolitan District of Quito during and after the COVID-19 pandemic. The findings revealed how local governments can reposition health systems to…

Antibiotics revolutionized modern medicine by treating various bacterial infections and decreasing morbidity and mortality rates. Despite these advantages, the overuse of antibiotics leads to the development of multidrug resistance (MDR) in bacterial species, particularly the ESKAPE pathogens, including Enterococcus faecium (E. faecium), Staphylococcus aureus (S. aureus), Klebsiella pneumoniae (K. pneumoniae), Acinetobacter baumannii (A. baumannii), Pseudomonas aeruginosa (P. aeruginosa), and Enterobacter species, which are resistant to lipopeptides, oxazolidinones, macrolides, tetracyclines, fluoroquinolones, β-lactams, polymyxins, a combination of β-lactam-β-lactamase inhibitor, carbapenems, and glycopeptides.1 The most critical pathogens globally that need to be combated with the more advanced antibiotics are P. aeruginosa, Enterobacter species, and A. baumannii, as these bacterial species are resistant to the last-line drug for multidrug-resistant bacteria, ie, carbapenem.2 The World Health Organization (WHO) states that the development of multidrug-resistant infections leads to a serious challenge to treatment methods, and affects global health, economy, and food security.3 The consequences of multidrug-resistant pathogens are given in Figure 1.
|
Figure 1 Associated outcomes of Multidrug resistance. Multidrug resistance can result in decrease in the efficacy of treatment, increase the risk of mortality rate, increase the treatment costs, prolong hospital stays, prolong illness. It rapidly develops in immunocompromised patients, and can lead to limited treatment options. Abbreviation: AMR, Antimicrobial resistance.
|
Therefore, there is an urgent need to develop more effective treatment modalities for MDR infections, as current antibiotics are often rendered ineffective against these rapidly evolving pathogens.4 The therapeutic challenge posed by MDR bacteria including ESKAPE pathogen demands innovative solutions to restore and enhance antimicrobial efficacy.5,6 In parallel with new treatment strategies, improving early detection and prevention methods remains critical for limiting the spread of resistance and enabling timely clinical intervention.7 Among emerging approaches, nanomedicine stands out as a versatile and promising avenue, offering targeted drug delivery, the ability to circumvent established resistance mechanisms, and the potential to simultaneously enhance both therapy and diagnostics for infectious diseases.8–11Nanoparticles are engineered, minute structures of a size range 1–100 nm, and are used as sensors, drug carriers, and antimicrobial particles that deal with AMR (Antimicrobial resistance) and even with bacterial biofilms. Nanoparticles is beneficial due to its ability to sustain release, enhanced penetration, targeted delivery, and enhanced solubility as compared to conventional therapeutic methods.12 Nanomedicine with intrinsic attributes, ie, to enhance the effectiveness of traditional antibiotics, is also available to deal with MDR bacteria. The nanoparticles are used as carriers for antimicrobial therapies, such as in the form of polymeric nanoparticles, dendrimers, and liposomes, collectively termed Nanobiotics,13 as well as used as an inherent therapy, such as zinc oxide (ZnO), titanium dioxide, nickel nanoparticles,14 copper nanoparticles, silver nanoparticles, and selenium nanoparticles.15 The carriers such as polymeric nanoparticles, liposomes, or dendrimer, are nanoscale delivery vehicles engineered to encapsulate and transport therapeutic agents (including antibiotics or peptides) directly to target sites of infection, thereby improving drug bioavailability, enhancing cellular uptake, and enabling precise targeting of pathogenic microorganisms.16–19 All of these nanoparticles have unique features that interact with the membrane components of bacterial cells, disturb biofilm, produce ROS (reactive oxygen species), and deliver antimicrobial agents with accuracy to the target.20
While nanoscale carriers such as polymeric nanoparticles, liposomes, and dendrimers are engineered to improve bioavailability and enable targeted delivery, not all nanoparticles inherently possess accuracy in tissue targeting. In practice, most nanoparticles administered systemically distribute throughout the body in a relatively conventional manner, with only a small fraction reaching the intended site due to biological barriers and clearance mechanisms. Achieving precise targeting requires additional modifications such as decorating the nanoparticle surface with specific ligands or optimizing particle size and surface characteristics to facilitate accumulation and uptake at the target cells or tissue.21–23 Even with such engineering, the efficiency of targeted delivery is often limited in clinical settings, representing a significant challenge for the translation of nanoparticle-based therapeutics.
Apart from therapeutic applications, nanoparticles increasingly play a central role in diagnostics, enabling the rapid identification of microbial pathogens and the detection of genetic determinants conferring antimicrobial resistance. Nanomedicine-based imaging techniques and biosensors allow for highly sensitive and specific molecular detection of resistance genes such as extended-spectrum β-lactamase (ESBL) genes, carbapenemase enzymes, and other genetic markers thus supporting targeted and effective treatment strategies.24–26 These diagnostic innovations are especially valuable in resource-limited healthcare settings, where rapid and accurate identification of resistant infections can improve patient outcomes.25,27 While nanomedicine-based diagnostics and therapeutics offer rapid, sensitive, and innovative approaches for managing multidrug-resistant infections, it is important to acknowledge that the majority of advanced nanoparticle technologies are currently costly to produce and require considerable technical infrastructure. As a result, their implementation has been largely limited to well-resourced healthcare systems, and widespread adoption in low-resource settings remains more aspirational than routinely achieved.12,28–30 Promising developments such as point-of-care (POC) nanodiagnostics, lab-on-chip devices, and microfluidic biosensors, are actively being optimized for affordability, simplicity, and robust function in resource-limited environments.31–33 Some nanoparticle-enabled POC platforms have already improved access to rapid diagnostics in remote or underserved regions by reducing reliance on centralized laboratories and facilitating earlier intervention.31,32
Despite these advances, the widespread implementation of nanoparticle-based diagnostics and therapeutics faces significant challenges. These include high production costs, technical complexities in maintenance, variability in batch-to-batch reproducibility, and stringent requirements for quality control.12,31–33 Furthermore, issues such as biocompatibility, potential toxicity, regulatory hurdles, and the demands of large-scale manufacturing continue to pose barriers to clinical translation and equitable deployment.29,34–36 Although the risk of resistance development to nanomedicine is currently perceived to be low, it remains an important consideration for long-term effectiveness. Therefore, while nanotechnology holds substantial promise for improving equity in infectious disease diagnosis and management worldwide, realizing its full clinical potential will require sustained innovation focused on cost-effectiveness, scalable production, rigorous regulatory approval, and practical application in diverse healthcare settings. This review provides a comprehensive overview of nanomedicine strategies aimed at combating multidrug-resistant infections.
This narrative review was conducted following principles based on the PRISMA guidelines for systematic reviews.37 We systematically searched databases including PubMed, Scopus, and Web of Science using the keywords: “nanomedicine”, “antimicrobial resistance”, “multidrug-resistant infections”, “nano-enabled therapy” and “translational prospects”.
The search interval was until April 2025 (inclusive), covering major publications from the last years. Only peer-reviewed articles in English were considered. Inclusion criteria were:
Exclusion criteria were editorials, conference abstracts, and non-English publications.
Two authors independently screened initial search results for relevance and quality. Disagreements were resolved through team consensus.
Nanomaterials have diverse physicochemical attributes that make them well-adapted for their use as antimicrobial agents. They have a surface charge, tunable size, functionalization potential, and a high surface area to volume ratio, making them capable of more precise interaction with the microbes, enhancing their therapeutic efficacy and solubility. Diverse types of nanoparticles are studied for their effects against multidrug-resistant microbes; each of them has specific functions in diagnostics and therapeutics.
The field of nanomedicine builds upon several decades of foundational research into nanomaterial synthesis, functionalization, and biological interaction. For example, early clinical adaptation of liposomal drug delivery systems such as liposomal amphotericin B (AmBisome) and liposomal doxorubicin (Doxil) highlighted the value of phospholipid vesicles for improving therapeutic efficacy and reducing toxicity in infectious disease and cancer management.38,39 Seminal work by Gregoriadis and Ryman first demonstrated the potential of liposomes for drug encapsulation and sustained release.40 In the context of antimicrobial resistance, classic studies showed that liposome-encapsulated antibiotics could target resistant strains more efficiently than free drugs.41–43
Liposomes are composed of a phospholipid bilayer in spherical form, and this bilayer encloses an aqueous core. They are biocompatible, biodegradable, and can encapsulate hydrophobic and hydrophilic drugs and act as a vehicle to carry antimicrobials. Liposomes enhance the antibiotics’ bioavailability, pharmacokinetics, precise delivery to the infection point, and reduce toxicity.44 An example of liposome-directed drug delivery is Doxil, in which doxorubicin is loaded via a PEGylated liposome. Passive targeting was employed to deliver the drug, and it relies on EPR (enhanced permeability and retention).45,46
Liposomes have better permeability, as evidenced by the gentamicin and vancomycin in liposomal formulations, which exhibit enhanced permeability and efficacy against methicillin-resistant Staphylococcus aureus (MRSA) and P. aeruginosa, allowing for sustained penetration into tissues and release of the drug. Surface modifications of liposomes, like ligand conjugation and PEGylation, facilitate active and passive targeting of liposomes to the infection site with accuracy and increase therapeutic effects.47
These nanoparticles can be synthesized from natural as well as synthetic polymers. The natural polymers include alginate and chitosan, and synthetic polymers include polycaprolactone (PCL) and poly lactic-co-glycolic acid (PLGA).48 These nanoparticles ensure drug release in a very controlled manner and can deliver therapeutic agents by protecting them from the degradation of enzymes, and it is also known for delivering multiple drugs at the same time.49
Natural polymers such as chitosan and alginate are commonly used to prepare polymeric NPs. Chitosan, for instance, is recognized for its high biocompatibility, biodegradability, and inherent antimicrobial activity due to its cationic properties.50–54 The chitosan have a cationic nature, due to which they have the ability to interact strongly with negatively charged bacterial membranes, leading to membrane disruption and antibacterial effects.49–51 These features enable chitosan NPs However, while natural polymer-based NPs offer bioactivity, they may also demonstrate higher immunogenicity and batch-to-batch variability, potentially limiting their reproducibility and scalability in clinical settings.50–54
By contrast, synthetic polymeric nanoparticles, formulated from PLGA, PCL, Polylactic acid (PLA), Polyvinyl alcohol (PVA), or Polyethylene glycol (PEG), are deliberately engineered to offer predictable degradation, lower immunogenicity, and finely tuned drug release profiles, which enhance their translational potential for clinical applications.52–56 Synthetic PNPs typically lack bioactivity unless specifically modified with targeting ligands, surface functionalities, or loaded agents, which can be tailored for specific therapeutic needs. Furthermore, the internal structure of PNPs, nanospheres (solid matrices) and nanocapsules (core-shell systems), directly affects drug encapsulation, stability, and release kinetics, thereby influencing efficacy and safety in biomedical settings.52–56A few antibiotics like rifampicin and ciprofloxacin are encapsulated by nanoparticles composed of PLGA, which increases the efficacy of these antibiotics for intracellular microbes like M. tuberculosis (Mycobacterium tuberculosis).57 The functionalization of these nanoparticles with different ligands like peptides and antibodies promotes their delivery to particular tissues or the bacterial strain.58
Rigorous characterization of polymer composition, particle size, surface charge, and morphology, using techniques such as dynamic light scattering, electron microscopy, and zeta potential analysis, is critical to ensure reproducibility, performance consistency, and safety in drug delivery and other biomedical uses.52–54,56 These nuanced differences between natural and synthetic polymeric nanoparticles highlight the importance of careful material selection and engineering for specific clinical applications.
3.3 Metallic and oxide nanoparticles Metallic nanoparticles, such as gold, silver, and copper nanoparticles, are intentionally synthesized nanoscale materials (1–100nm) known for their unique physicochemical, electronic, and catalytic properties. These engineered metallic nanoparticles exhibit pronounced antimicrobial activity against a wide spectrum of multidrug-resistant bacteria, including both Gram-positive and Gram-negative strains,59 through mechanisms such as membrane disruption, induction of ROS, and inhibition of microbial metabolic pathways.36,60–62 In addition to metallic forms, metal oxide nanoparticles including ZnO, copper oxide (CuO), and iron oxide (Fe₂O₃) nanoparticles, have emerged as potent antimicrobial agents. Their bactericidal effects are attributed to their ability to generate ROS, release metal ions, and physically interact with bacterial membranes, ultimately disrupting cellular integrity and function.63–68
Oxide nanoparticles, such as ZnO, have demonstrated broad-spectrum efficacy, with studies showing strong antimicrobial activity against both Gram-positive (eg, S. aureus, Bacillus subtilis) and Gram-negative (eg, E. coli, P. aeruginosa) pathogens.63,65 The antimicrobial action is size-dependent, with smaller particle sizes generally exhibiting greater bactericidal potency due to higher surface reactivity. The development and biomedical application of both metallic and metal oxide nanoparticles represent important advances in the fight against multidrug-resistant infections.
Due to the inert nature of this metal, the AuNPs perform their function in the functionalization with peptides, antibiotics, and ligands. They are involved in the delivery of different drugs and are used most effectively for diagnostic purposes due to the unique optical attributes of this metal.69
AuNPs exist in various forms, distinguished by their size, shape, surface functionalization, and composition, each imparting unique biological activities and limitations.60,70
The most common types include spherical colloidal AuNPs, nanorods, nanocages, nanostars, and core-shell structures, which are synthesized for specific biomedical applications ranging from imaging to drug delivery and photothermal therapy.70,71 For the treatment of chronic infections, AuNPs are used as cargo for the transport of dual therapeutic compounds having anti-inflammatory and antimicrobial properties. The surface of these nanoparticles is conjugated with dexamethasone, which is an anti-inflammatory compound used to prevent tissue damage. These nanoparticles have the potential to treat the infection of intracellular pathogens like M. tuberculosis.72
Spherical AuNPs are widely used for their simplicity and stable optical properties. Nanorods and nanostars are engineered for enhanced plasmonic effects suitable for diagnostic sensors and cancer therapy due to their tunable absorption in the near-infrared range.70,71,73
AuNPs generally exhibit high biocompatibility and low immunogenicity compared to other metallic nanoparticles, but their surface chemistry, especially when modified with biomolecules or polymer, can influence immune responses and cellular uptake.73,74
For example, AuNPs conjugated with antibodies and antigens have been shown to both amplify and modulate immune reactions, highlighting their potential as vaccine carriers or diagnostic adjuvants, but also indicating a need for careful immunological assessment.71,73,74
Limitations of AuNPs include size-dependent cytotoxicity, unwanted accumulation in tissues, and possible oxidative stress at very small diameters (<2 nm), necessitating comprehensive biocompatibility and safety evaluations prior to clinical application.70,74
Despite their relatively inert and stable nature, gold nanoparticles may still cause cell toxicity if used at high concentrations or with inappropriate surface ligands, and standardization challenges remain in regulatory frameworks for their safe biomedical deployment.74,75
Furthermore, AuNP-based nanocomposites and hybrid structures such as AuNPs loaded with drugs, peptides, or genetic material, can be tailored to enhance targeted therapy or bioactivity, but may inherit limitations or safety concerns from the combined materials.70,73 Overall, the selection and engineering of specific types of AuNPs must be guided by a balance between desired bioactivity, minimal immunogenicity, optimal biocompatibility, and application-specific limitations.70,74,75
The ZnO NPs exist in various forms, each exhibiting distinct physicochemical properties and biomedical characteristics depending on synthesis routes, particle size, surface charge, and functionalization. ZnO NPs can be synthesized by chemical methods (such as sol-gel and precipitation techniques) or by green methods using plant extracts or biopolymers. Chemically produced ZnO NPs typically possess smaller crystallite sizes, while green-synthesized ZnO NPs tend to have higher crystallinity and purity.76–79
The bioactivity of ZnO nanoparticles is closely linked to their ability to generate ROS, which underpins their prominent antibacterial, antifungal, antineoplastic, wound healing, and biosensor functions. These nanoparticles have photocatalytic activity, as the ROS increase when exposed to light. They are involved in the degradation of bacteria’s biofilms using oxidative stress.80
Smaller ZnO NPs and those with positive surface charges show heightened cellular internalization and antibacterial effectiveness but may also display increased cytotoxicity when compared to their larger or negatively charged counterparts. ZnO nanoparticles are used for gram-positive as well as gram-negative bacteria like S. aureus, P. aeruginosa, and E. coli. As they show photocatalytic activity under ultraviolet, they are a potential agent for coating on food packaging and medical types of equipment. They are biocompatible, low-cost, and scalable agents for the management of antimicrobial-resistant infections.81
Immunogenicity and toxicity of ZnO NPs are variable and depend on several factors. ZnO NPs with different sizes and charges have demonstrated a range of immunotoxic effects, including induction of inflammation, suppression of natural killer cell activity, and alterations to cytokine profiles, sometimes leading to immunosuppression in vivo. Specific studies have reported oxidative stress, cytotoxicity, DNA damage, and organ inflammation (lung, liver, kidney, heart, and brain), which are concerns for clinical translation.82–84 The surface charge is especially critical: positively charged ZnO NPs have shown higher cytotoxicity and enhanced cellular uptake than negatively charged NPs.82–84
Despite FDA approval of bulk zinc oxide as generally recognized as safe (GRAS), the nanoscale form poses unique toxicological risks due to high solubility and increased biological reactivity. These risks highlight limitations in clinical use, including potential pulmonary toxicity (by inhalation exposure), genotoxicity, and the need for careful dose selection.82,83
In summary, distinctions in synthesis approach, particle size, charge, and functionalization yield ZnO NPs with varying immunogenicity, bioactivity, and limitations, necessitating rigorous characterization and risk assessment for biomedical applications.76–78,82–84
The AgNPs are a diverse class of nanomaterials produced using various synthesis methods, including physical, chemical, and biological (green) routes, resulting in distinct particle sizes, shapes, and surface properties.85–87 Each synthesis method can influence the immunogenicity, stability, and bioactivity profile of the resulting AgNPs; for instance, biologically synthesized AgNPs may have lower environmental toxicity and enhanced biocompatibility compared to those synthesized chemically.85–88 The metallic nanoparticles are stable, and even their low concentration is effective, but there are a few concerns with regard to their biodistribution, cytotoxicity, and aggregation in tissues for the long term. The plant-based synthesis of these nanoparticles makes these particles biocompatible and with a lower cytotoxicity level.89
Silver nanoparticles have been investigated for their antimicrobial properties since the pioneering studies by Sondi and Salopek-Sondi in 2004, which demonstrated broad-spectrum bactericidal activity through membrane disruption and ROS generation.90 Subsequent research study provided mechanistic insight into the size-dependent antimicrobial effects of silver nanoparticles.91 AgNPs exhibit broad-spectrum antimicrobial activity against bacteria, fungi, and viruses through multiple mechanisms, primarily the release of silver ions (Ag⁺), generation of ROS, and disruption of microbial membranes.85,92–94 They are used for coatings, and wound dressings, and are used along with antibiotics for the more enhanced synergic attributes.95,96
Their bioactivity is size-, shape-, surface charge-, concentration-, and time-dependent, with smaller sizes (<10 nm) showing enhanced antibacterial effects due to a higher surface-area-to-volume ratio. Spherical AgNPs have generally been observed to exhibit superior antibacterial efficacy compared to other shapes, highlighting the importance of morphology for bioactivity.85,92–94
However, one limitation includes potential cytotoxicity and immunogenicity. AgNPs may accumulate in tissues and cells, induce oxidative stress, and upregulate cellular markers related to inflammation and stress responses, which raises concerns for chronic or systemic exposure.85,86,93,94 Moreover, long-term and improper use of AgNPs can induce microbial resistance through mechanisms such as increased expression of efflux pumps, biofilm formation, and genetic adaptation, including mutations allowing bacteria to detoxify or expel silver ions.85,86,93,94
Batch-to-batch variability in AgNPs, especially those synthesized using biological sources, can affect both biological activity and safety profiles, impacting scalability and reproducibility for biomedical use. Adjusting surface stabilizers, such as polyvinylpyrrolidone (PVP) or citrate, can help modulate immunogenicity and improve dispersion, but may also change bioactivity and biocompatibility.85,87,88 Recent work has even described the development of novel sub-nanometer silver particles (silver Angstrom particles) which show better biological activity and lower toxicity compared to standard AgNPs.85
Dendrimers are a class of highly branched, tree-like macromolecules that can be categorized by their core structure and surface functionality, leading to a range of biomedical properties.97,98 Polyamidoamine (PAMAM) dendrimers are among the most widely studied, known for their regular architecture and ability to be tailored for drug encapsulation or conjugation, but higher-generation and cationic PAMAM dendrimers demonstrate increased cytotoxicity and immunogenicity compared to lower-generation or neutral/anionic types.97,99 Polypropylene imine (PPI) dendrimers similarly offer versatile functionalization opportunities and substantial bioactivity, though their toxicity also correlates with generation and terminal group chemistry, requiring careful design for safe medical use.97,100 Carbosilane dendrimers, featuring silicon-based backbones, have lower toxicity and immunogenicity than cationic PAMAM dendrimers, making them more suitable for biological applications.97 Peptide dendrimers represent another type, engineered to enhance biocompatibility, and minimize the immune response, while maintaining functional versatility for gene delivery or antimicrobial use.97,99
Dendrimers were first explored as drug carriers, with applications for antimicrobial agent delivery soon following.101–104 Early research in this domain revealed their potential for high drug loading and targeted release against resistant pathogens.105 The bioactivity of dendrimers such as membrane penetration and targeted drug delivery, largely depends on their surface groups: cationic dendrimers can disrupt cell membranes via electrostatic interaction, leading to apoptosis, whereas anionic or neutral terminal groups decrease cytotoxicity and immunogenicity.97,99,106,107 Despite their promise, limitations of dendrimers include cytotoxicity (particularly for cationic types), potential immunogenicity, restricted permeability for higher generations, and complex or costly synthesis and purification procedures. Many biomedical dendrimers are modified to balance activity with safety, such as PEGylation to improve solubility and circulation or surface neutralization to reduce immunogenicity.97,99,106,107.
Rigorous assessment of dendrimer type, generation, and functionalization is critical to optimize biocompatibility, minimize side effects, and ensure reproducibility for diagnostic or therapeutic use.97,99,107
Furthermore, the engineering of dendrimers can improve the bioavailability of insoluble drugs as well as solubilize these drugs. These particles are engineered to respond to external stimuli like pH or respond in the presence of a specific enzyme, which makes their delivery targeted and multidrug-resistant infection treated more efficiently. However, a few concerns about high-generation dendrimers are about their complexity and toxicity, which limit their clinical usage.108
Various types of carbon-based nanostructures include fullerenes, carbon nanotubes (CNTs), graphene oxide (GO), reduced graphene oxide (rGO), graphene quantum dots (GQDs), carbon nanofibers, and carbon dots (CDs), each with distinct characteristics impacting their biomedical applications and antibacterial activity.109–114 Their synthesis may rely on top-down processes (eg, exfoliation of graphite for graphene and GO) or bottom-up approaches (eg, vapor deposition or chemical synthesis for CNTs, fullerenes, and carbon dots). Chemical and physical functionalization through covalent attachment of functional groups, noncovalent interactions, heteroatom doping, and bioconjugation, can improve dispersibility, targeting capacity, drug loading, and biological compatibility.110–115 The development of carbon-based nanomaterials, such as graphene oxide and carbon nanotubes, was marked by discoveries of their ability to disrupt microbial membranes and generate oxidative stress, as evidenced by the previous studies.116,117
The immunogenicity and cytotoxicity profiles of carbon-based nanostructures vary considerably with surface chemistry, particle size, and degree/type of functionalization.110,115 Recent reviews indicate functionalization generally reduces immunogenicity compared to raw materials, makes nanoparticles more biocompatible, and can tailor bioactivity. However, aggregation, sharp edges (eg, GO sheets), and residual catalysts may contribute to immune activation or cytotoxicity and thus represent critical limitations in some biomedical contexts.110,115
Distinct antibacterial mechanisms are observed: for instance, direct contact between CNTs, GO, or fullerenes and the bacterial membrane can disrupt membrane structure, induce metabolic compromise, and provoke cell death. Mechanisms include oxidative stress resulting from ROS generation, mechanical disruption of membranes (especially for 2D structures like GO), and interference with energy metabolism or DNA activity.109,110,114
The size and surface charge of carbon-based nanostructures are key determinants of antibacterial efficacy.110,113 Smaller particles generally have a larger surface-to-volume ratio, supporting enhanced interactions with bacteria; positively charged surfaces may bind vigorously to negatively charged bacterial membranes, boosting membrane disruption. A recent study showed that smaller carbon dots demonstrated superior antibacterial activity owing to efficient membrane interaction and ROS production.110,113
Carbon-based nanoplatforms may be constructed from different foundational materials pristine or functionalized carbon nanotubes, graphene oxide, fullerenes, nanodots, or hybrid composites, that influence their antibacterial mechanisms.110,114,118,119 For example, CNTs generally act via physical piercing and cell aggregation, GO by oxidative stress and membrane slicing, fullerenes primarily via ROS generation and metabolic interference, and carbon dots by membrane interaction and photo-induced processes.110,114,118,119
The choice of carbon nanomaterial, its size, surface charge, functionalization, and hybridization all determine its antibacterial action, immunologic profile, and suitability for a given clinical setting.110,113–115,119
The GO nanostructures encompass a variety of forms including nanosheets, quantum dots, nanoribbons, and hybrid composites such as GO decorated with metal nanoparticles or embedded in polymeric matrices. Each structure possesses distinct physical and chemical characteristics determined by synthesis methods such as chemical exfoliation, Hummers’ method, or electrochemical approaches, which control the number of layers, lateral size, oxidation degree, and defect density, ultimately impacting antibacterial activity.116,120–122 GO nanostructures can be further functionalized by covalent grafting, non-covalent adsorption, and conjugation with metals (eg, silver, nickel, copper), polymers, and biomolecules, modifying their dispersibility, targeting, and biological interactions.116,120–122
These functionalization strategies not only optimize the bioactivity and stability of GO but also directly influence immunogenicity, cytotoxicity, and overall safety profiles, determining feasibility for therapeutic applications.116,120,123,124 However, the use of GO nanostructures faces certain limitations: natural variability in size and composition, possible aggregation, and challenges in reproducible large-scale manufacture and toxicity control, including potential induction of reactive oxygen species and inflammation.116,120,123,124 The basic characteristics of GO such as sheet size (smaller sheets may induce more oxidative stress and membrane disruption; larger sheets tend to physically trap bacteria), surface charge (typically negative, but can be modified), and oxidation level, all markedly affect antibacterial mechanisms and efficacy. Higher defect density and sharp edges can physically puncture bacterial membranes, while charge interactions facilitate wrapping or entrapment, which may be bacteriostatic or bactericidal depending on context.116,120,123,124 These sheets, along with antibiotics, are used to kill multidrug-resistant bacteria.125
Different materials can be incorporated to assemble GO nanoplatforms, such as AgNPs, nickel colloidal clusters, and copper nanoparticles, which impart synergistic activity and extend the antibacterial spectrum against resistant pathogens.116,121–124 Their mechanism of action often combines multiple pathways including membrane disruption by sharp nanosheets, ROS-induced oxidative stress, entrapment and metabolic interference, or metal ion release from the composite, tailored by the nature of each GO-based nanostructure. For example, functionalization with AgNPs enhances bactericidal potential via both physical and chemical means, whereas hybridization with polymers or biomolecules can reduce cytotoxicity and improve biocompatibility.116,121–124
Graphene oxide showed effective results against K. pneumoniae and MRSA. It is used for the creation of nanocomposites due to its synergistic effect along with other nanoparticles and antibiotics. Due to its dispersion ability in water and biocompatibility, it is used for drug delivery, to disrupt biofilms, and for wound dressings.126
The CNT nanostructures include single-walled carbon nanotubes (SWCNTs), multi-walled carbon nanotubes (MWCNTs), and double-walled carbon nanotubes (DWCNTs), each with distinct structural characteristics and synthesis methods.127–129 SWCNTs consist of a single graphene cylinder and are typically synthesized via chemical vapor deposition (CVD), laser ablation, or arc discharge, whereas MWCNTs have multiple concentric graphene cylinders and may be produced by CVD or nanocycle production techniques. Functionalization of CNTs, such as covalent attachment of biomolecules, polymers, or drugs to their surface, improves solubility, dispersion, and reduces cytotoxicity while enabling targeted antibacterial activity.127–129
CNTs have electrical conductivity and effective mechanical strength; they have a needle-shaped appearance that allows them to penetrate inside the bacterial membranes, causing leakage of cytoplasmic content and ultimately cell death. They can overcome efflux pumps and targeted delivery of antibiotics, both single-walled and multi-walled CNTs can show antimicrobial properties along with other metal ions. They act as the major component of biosensors and also carry drugs. The carbon nanotubes penetrate the membranes and disrupt the cellular processes.130
The immunogenicity and bioactivity of CNTs are closely linked to their surface chemistry, physical properties, and degree of functionalization.128,129 While pristine CNTs have potent bioactivity, they may exhibit cytotoxicity and provoke inflammatory responses; functionalized CNTs, such as those with carboxyl (-COOH) or amine (-NH2) groups, tend to show reduced toxicity and improved biocompatibility.128,129 Limitations commonly encountered include aggregation in biological media, oxidative stress induction, inflammation, and batch variability arising from differences in synthesis and surface characteristics.128,129
Basic physicochemical characteristics, notably diameter, length, and surface charge, substantially impact their antibacterial performance. SWCNTs with smaller diameters and higher surface areas typically display stronger antibacterial activity due to enhanced membrane interaction and disruption, acting as nano-darts that physically penetrate bacterial cell walls and induce leakage of intracellular content.127,131,132 The length of CNTs also influences toxicity shorter tubes have greater density of open ends, increasing antimicrobial effects, while longer tubes tend to have lower bacterial toxicity. Surface charge governs electrostatic interactions with the negatively charged bacterial membranes, promoting cell adhesion, membrane potential reduction, and antibacterial potency.127,131,132
Various materials can be used to assemble carbon nanotube-based nanoplatforms, including pristine CNTs, polymer-CNT composites, metal-oxide-functionalized CNTs, and drug-conjugated CNTs. These platforms operate through mechanisms such as direct physical membrane disruption, generation of ROS, wrapping and entrapment of the microbes, and interference with metabolic pathways.133,134 Metallic CNTs often display higher antibacterial activity than semiconducting ones, owing to differences in electron transfer and ROS generation. The antibacterial effect may also be further enhanced by light activation, which induces additional ROS in bacterial cells.133,134 All these factors; CNT type, synthesis/functionalization, physicochemical properties, and the selected platform material, contribute to the spectrum, potency, and safety profile of CNT-based antibacterial nanostructures.127–129,132,134
There are several types of fullerenes, including the classical C60 (buckminsterfullerene), C70, endohedral fullerenes (fullerenes containing metal atoms or clusters inside the cage), and functionalized derivatives such as fullerols (hydroxylated fullerenes) and amino fullerenes.135–137 The synthesis of fullerenes commonly involves arc discharge, laser ablation, or chemical vapor deposition; further chemical functionalization can be applied to alter solubility, add targeting ligands, or improve bioactivity, for example by adding amino, carboxyl, or hydroxyl groups.135–137 Functionalization impacts immunogenicity, hydrophobicity, biocompatibility, and the ability to interact with biological systems, which in turn changes their antimicrobial and cytotoxic profiles.135–137 Although fullerenes and their derivatives exhibit bioactivity, some forms may induce inflammatory responses or cytotoxicity depending on dose, surface chemistry, and aggregation, which are considered limitations for biomedical uses.135–137
Basic characteristics such as particle size and surface charge significantly impact antibacterial activity.110,138,139 Smaller fullerenes possess larger surface areas, facilitating adsorption onto bacterial membranes and transport into cells, thus enhancing bactericidal effects. Cationic (positively charged) fullerene derivatives show greater efficacy against Gram-negative bacteria, which have negatively charged cell surfaces, due to strong electrostatic interactions that improve cell membrane disruption and bacterial death. In contrast, anionic or neutral fullerenes are often less effective for the same reason.110,138,139
The material and mechanism of action of fullerene-based nanoplatforms also vary. Pure fullerenes act primarily by generating ROS under irradiation, causing oxidative stress, membrane disruption, protein denaturation, and DNA damage in target bacteria.110,135,138,140,141 Furthermore, the C60 nanoparticles were reported photodynamic and antioxidant properties. They have a complex cape cage-shaped structure with singlet oxygen and release ROS upon photoactivation. They are involved in killing microbes by oxidative stress and conjugated with antibiotics for targeted delivery.142
Water-soluble or peptide-conjugated fullerene derivatives can enhance photodynamic efficacy and target multiple bacterial species, including multidrug-resistant strains, via improved membrane interaction and ROS production. Cationic, amphiphilic, or metal-coordinated fullerene nanomaterials possess further antibacterial advantages, including disruption of bacterial energy metabolism and increased permeability to antibiotics.110,135,138,140,141 The assembly of nanoplatforms using combinations of fullerenes and other materials such as metals, peptides, and polymers, can allow for synergistic antibacterial actions and broaden pathogen coverage.110,135,138,140,141
The main nanomaterials employed for the treatment of multidrug-resistant infection, along with their composition, mechanism of action, pros and cons, and currently used model, is shown in Table 1.
 |
Table 1 Nanomaterials and Their Attributes
|
Different nanomaterials, along with their antimicrobial properties and mode of action, is demonstrated in Figure 2.
 |
Figure 2 Different Nanomaterials and their antimicrobial properties. Abbreviation: ROS, reactive oxygen species.
|
The route of nanoplatform administration fundamentally influences the therapeutic efficacy and safety profile of treatments for infectious diseases, especially those caused by multidrug-resistant pathogens. Local application strategies such as topical gels for skin infections, wound dressings incorporating nanoparticles, inhalable aerosols for respiratory conditions, or intravaginal delivery for gynecologic infections, maximizing the concentration of active agents at the infection site. This targeted exposure enhances antimicrobial activity, limits systemic side effects, and reduces the risk of off-target tissue toxicity.150–153 Moreover, localized delivery enables the use of higher drug doses, circumvents some mechanisms of systemic drug resistance, and has shown particular success in difficult-to-treat superficial or mucosal infections.152,154
In contrast, systemic administration including intravenous, oral, or intramuscular routes, is required for treating deep-seated, disseminated, or multi-organ infections where local application is impractical or ineffective. While systemic delivery allows therapeutic nanoplatforms to reach multiple or inaccessible sites, it is frequently challenged by biological barriers such as rapid clearance by the mononuclear phagocyte system, variable biodistribution, renal or hepatic elimination, and accumulation in non-target organs.21,23,155,156 These factors can dilute drug concentration at the site of infection and elevate the risk of adverse effects. Achieving specific targeting and optimal drug levels in affected tissues therefore remains a major limitation for systemically administered nanomedicines.
Recent advances in nanoparticle engineering such as surface modification with ligands for enhanced targeting, stealth coatings (eg, PEGylation) to improve circulation time and evade immune clearance, and stimulus-responsive systems that release drugs in response to local microenvironmental cues, are actively under investigation to address these challenges.154,156 The decision between local and systemic nanoplatform delivery should be individualized according to the infection’s location, severity, patient-related factors, and properties of the nanomaterial, with ongoing research focused on improving both approaches.
Nanomaterials are employed against MDR bacteria due to their ability to act through multiple antimicrobial mechanisms, providing a broader spectrum of activity than conventional antibiotics.157,158 Unlike conventional antibiotics, which typically target bacteria via one or two specific biochemical pathways, nanomaterials exert their killing effect using a combination of biochemical, molecular, and physicochemical routes.158,159
This multi-modal action includes membrane disruption, generation of oxidative stress through ROS, intracellular penetration of antimicrobial agents, inhibition of biofilm formation, controlled release of therapeutic agents, and synergism with antibiotics.157,160–162 Disruption of the bacterial membrane by nanoparticles may occur via direct interactions such as electrostatic attraction or adsorption of metal ions onto membrane components and can result in leakage, destabilization, and cell death.161
ROS generation by nanoparticles (such as silver, copper, zinc oxide, titanium dioxide) leads to oxidative damage of lipids, proteins, and DNA, further compromising bacterial viability and driving apoptosis or cell death.160,161,163 Some nanoparticles penetrate into bacterial cells by diffusion or endocytosis, where they interact with intracellular targets including nucleic acids and enzyme systems, disrupting essential cell processes.157,161 The ability of nanomaterials to inhibit biofilm formation is particularly valuable against MDR strains, as nanoparticles can disrupt established biofilms and prevent their regeneration by interfering with bacterial signaling and extracellular matrix components.157,158
Nanoparticles can act as drug carriers, enabling the controlled release of antimicrobial agents, which enhances efficacy and reduces the likelihood of resistance development.158 Finally, combining nanomaterials with conventional antibiotics creates synergistic effects, increasing antimicrobial activity and bypassing resistance mechanisms that often thwart monotherapies.157,158
One of the most common antimicrobial mechanisms employed by nanoplatforms is disruption of the bacterial cell membrane. Bacterial membranes are characteristically negatively charged due to the presence of teichoic acids in gram-positive bacteria and lipopolysaccharides in gram-negative strains, making them particularly vulnerable to positively charged (cationic) nanoparticles.160,164,165 Cationic nanoparticles such as chitosan NPs, dendrimers, and metallic nanoparticles like ZnO and silver oxide are able to interact electrostatically with the bacterial membrane; these interactions lead to destabilization of membrane structure, increased permeability, and subsequent cell lysis with leakage of cellular contents.160,164–166
Experimental studies have demonstrated that chitosan-grafted oligolysine chains and branched polyethyleneimine-functionalized silver nanoclusters exert strong bactericidal activity by selectively binding to anionic bacterial membranes, causing membrane injury and cell death while sparing mammalian cells.49,165,167–172 Mechanical disruption is another pathway: nanomaterials such as graphene oxide and carbon nanotubes physically interact with the bacterial membrane, sometimes piercing or rupturing the membrane and releasing cellular content, which directly damages the cell and reduces the risk of resistance development.173–175
Direct contact between nanoparticles and bacterial cell membranes has been identified as a primary mechanism for cytotoxicity; destructive interactions by carbon-based or inorganic engineered nanoparticles result not only from electrostatic attachment but also from physical tearing and oxidative or chemical stress.145,175,176 Overall, these mechanisms, electrostatic disruption and mechanical damage, enable different nanomaterials to bypass traditional resistance pathways and exert powerful, multi-modal antimicrobial effects.145,160,165,174,175
Metallic nanoparticles, such as ZnO nanoparticles, silver oxide nanoparticles, and titanium oxide nanoparticles, as well as carbon-based nanostructures, are known to possess photocatalytic properties that enable them to generate ROS.177,178 The generation of ROS can occur spontaneously in biological environments or be stimulated by photocatalysis, leading to the formation of molecular species such as superoxide anion (O2−), hydroxyl radical, hydrogen peroxide, and singlet oxygen (1O2).177,179 These highly ROS induce oxidative stress, which damages bacterial cells by disrupting essential biomolecules including proteins, lipids, and nucleic acids; this results in denaturation of proteins, lipid peroxidation of membranes, and degradation or fragmentation of bacterial DNA, rendering the microbes non-viable.177,180
The AgNPs, for instance, can bind to DNA and phosphorus-containing biomolecules, causing strand breaks and interfering with the replication process, ultimately leading to bacterial cell death.177,181
Furthermore, antibiotics delivered via nanoparticles can interact with the bacterial cell, inhibit the electron transport chain, interfere with efflux pumps, degrade crucial enzymes, destabilize ribosomal machinery, and inhibit protein synthesis, collectively leading to multifaceted disruption of bacterial viability, as shown in Figure 3.177,182 Carbon-based nanoparticles like GO and fullerenes also produce ROS, especially upon photoactivation, which adds to their antimicrobial capabilities against targeted infections and biofilms, affecting bacterial survival and contributing to biofilm disruption.177,183
 |
Figure 3 Nanoparticle (NP)-mediated antibiotic delivery and cell disruption using the oxidative stress mechanism. (1) Functionalized NPs encapsulate or conjugate antibiotics, ensuring enhanced stability and site-specific delivery to bacterial cells. (2) NPs attach to the bacterial cell wall and membrane, facilitating antibiotic entry and enhancing penetration. (3) NPs disrupt the cell wall, membrane, enzymes and mitochondria, destabilization of ribosomes and inhibition of protein synthesis, inhibit electron transport chain, damage efflux pumps, and generate reactive oxygen species to induce oxidative stress. (4) The synergistic mechanisms culminate in irreversible bacterial killing, offering a dual-therapeutic approach. Abbreviations: NPs, nanoparticles; NAb, nanoparticle albumin bound.
|
Few nanoparticles can penetrate within cells by the process of passive diffusion or endocytosis, and it depends upon the charge, size, and hydrophobic nature of the particle. After internalization, these nanoparticles interact with the different targets inside the cell. The metallic nanoparticles, such as silver nanoparticles, interact with the DNA and disrupt the process of replication and transcription.184 Few metallic and polymeric nanoparticles interact with the protein synthesis unit, ie, ribosomes and ribosomal RNA, disrupt other translation factors, and inhibit protein synthesis. The thiol group (-SH) containing nanoparticles interacts with the bacterial enzymes or inhibits the binding to the cofactor of the enzyme by replacing it, hence deactivating the enzyme activity as shown in Figure 4.185
 |
Figure 4 Penetration of silver nanoparticles to inhibit protein synthesis to combat multidrug resistant infections.
|
The silver ion of the silver nanoparticle interferes with the function of the respiratory chain enzyme and reduces the production of adenosine triphosphate (ATP).186 Nanoparticles are also involved in the disturbance of normal homeostasis of the cell by disrupting ion transportation through nanopore formation. These multiple strategies of nanoparticles make them an effective candidate for combating multidrug-resistant infections.187
One of the most beneficial features of the nanoparticles is their capability to encapsulate antimicrobial drugs and release them in a very controlled manner at a specific target.188 Different nanomaterials like liposomes, dendrimers, and polymeric nanoparticles like chitosan and PLGA are engineered to release the therapeutic drugs in response to specific cellular stimuli such as enzymes, pH, temperature, or some oxidation-reduction condition at the site of microbial infection.189
The active targeting of these nanoparticles is done by modifying the surface of nanoparticles with antibodies, and aptamers having some specific biomarkers or peptides. The passive targeting depends on the EPR effect, where some infected site allows the aggregation of these nanoparticles.190 The targeted delivery has benefits like enhanced bioavailability, and reduced toxicity, and ensures the maximum concentration of the microbial agent at the targeted site.
Biofilms are microbial cell communities aggregated in extracellular polymeric substance (EPS). It is very difficult to disrupt a biofilm as antibiotics are not capable of penetrating; also, the internal environment of the biofilm is altered, and slow-growing cells are present within this network.191 Nanomaterials can penetrate these complex biofilms in several ways, such as by physical penetration into biofilms, as nanoparticles have a small size and can penetrate better than traditional antibiotics. Some engineered nanoparticles carry specific enzymes like proteases and DNases, or they may have the ability to generate ROS, which can degrade EPSs and make the individual bacteria susceptible.192
Few nanoparticles can interact with the cell signaling pathways, and these pathways are essential for the maintenance of biofilms.193 The nanoparticles release the antimicrobials to the core of the biofilm, hence destroying it more effectively than traditional antibiotics. In P. aeruginosa and S. aureus, the biofilm formation is inhibited by chitosan and graphene oxide.194
Another important approach is the transport of traditional antibiotics with nanoparticles by engineering. This synergism of nanoparticles with traditional antibiotics enhances the efficacy of antimicrobial treatment. The nanoparticles disrupt the cell membrane and promote antibiotic uptake; they also protect the antibiotic from degradation, and they reverse the resistance mechanism by altering the target sites and by efflux pumps.195
Several studies supported the fact that antimicrobial activity is enhanced when antibiotics are transported via nanoparticles, like gold or silver nanoparticles and dendrimers. As in the case of vancomycin encapsulated with liposome and tetracycline in silver nanoparticles, it showed enhanced antimicrobial activity against MRSA and E. coli.196 Tetracycline (and other antibiotics) creates a TET–AgNP complex that more strongly binds to Salmonella typhimurium (≈21% increased Ag binding) and enhances Ag⁺ release (≈26%), producing a local Ag⁺ burst at the cell envelope that disrupts cellular function and prevents growth as shown in Figure 5.
 |
Figure 5 Synergism of silver nanoparticles with antibiotics to inhibit MDR Salmonella typhimurium DT 104. (1) Pathway I Direct attachment of AgNPs to the bacterial surface, leading to increased membrane binding and Ag⁺ ion release. (2) Pathway II (Minor): Formation of a TET–AgNP complex that penetrates the bacterial cell, interacting with intracellular targets and disrupting cellular processes. (3) Pathway III: Tetracycline molecules, either free or complexed, interact with ribosomes to inhibit protein synthesis, while AgNPs potentiate this effect by damaging the ribosomal machinery.
|
With the emergence of multidrug-resistant bacteria, there was a need to transport these antimicrobials to the infection site. Nanoparticles are engineered to act as a vehicle to transport these antimicrobial components.197 These nanoparticles encapsulate, protect, and transport these antimicrobial components to the targeted site. These nanocarriers enhance therapeutic efficiency by improving stability, and solubility, and reducing the toxicity and degradation of antimicrobials. The two major strategies to transport the antimicrobials to the targeted site are active and passive targeting.198 Nanoparticles also neutralize the toxicity caused by a few antibiotics, such as colistin, an antibiotic also has some nephrotoxic effects when given alone, but when colistin is encapsulated with liposomes, a reduction in nephrotoxicity was observed.199
Other nanomaterials like chitosan and dendrimers promote the penetration of the drug into bacterial cells, and targeted therapeutic effects can be achieved with relatively low concentrations of the drug.200 The encapsulation of the antibiotics with nanocarriers protects the drug from degradation and improves the efficacy of the treatment. Nanocarriers are also employed for the combined therapies, as it is loaded with two or more drugs to find out the synergic polymeric effect against polymicrobial infections.182 The nanocarriers overcome the traditional limitations, like toxicity, and also improve pharmacokinetics.201
Composite and hybrid nanomedicine systems, characterized by the integration of two or more bioactive components such as antibiotics, peptides, metals, enzymes, and polymers into a single nano-platform, are transforming the management of MDR infections. By leveraging synergistic antibacterial actions, these platforms expand pathogen coverage and enable significant dosage reductions, thereby lowering toxicity and potentially slowing the emergence of resistance.177,202
Recent studies have demonstrated that combination nanoparticle platforms, such as silver–platinum (AgPt) nanohybrids, exert potent synergistic antibacterial and antibiofilm effects when paired with conventional antibiotics. For example, AgPt nanohybrids combined with streptomycin, rifampicin, or ampicillin have led to markedly reduced minimum inhibitory concentrations and improved biofilm eradication against E. coli, S. aureus, and P. aeruginosa.203 These outcomes highlight the potential of metal–metal hybrid nanoparticles as adjuvants to conventional drugs.
Hybrid metallic nanoparticles conjugated with antibiotics or antimicrobial peptides have also been shown to surpass the efficacy of single-agent therapies, effectively suppressing MDR pathogen growth and preventing biofilm formation.204,205 Polymeric nanogels and dendrimer-based composites designed to co-deliver multiple drugs or enzymes have demonstrated broad-spectrum activity against drug-resistant strains, disrupting established biofilms and improving antibiotic penetration.206,207 Furthermore, composite nanomedicines that incorporate ZnO or CuO nanoparticles alongside antibiotics achieve deeper antibacterial action, including up to six‑fold reductions in drug dosage, with significant efficacy against MDR P. aeruginosa.208
Hybrid nanosystems are increasingly being engineered for precision combination therapy, allowing either simultaneous or staged release of agents with complementary mechanisms of action. Examples include polymer–lipid hybrid nanoparticles loaded with antibiotics and antimicrobial peptides, as well as nanofiber membranes combining metallic nanoparticles with antibiotics for long-term barrier effects.204,205 Such architecture not only enhances bactericidal synergy but can also improve local retention and support chronic infection management.
The mechanistic advantages of these composite strategies stem from their capacity to attack bacteria through multiple pathways including cell membrane disruption, metabolic interference, ROS generation, and efflux pump inhibition, making resistance emergence less likely.202,209 The integrated delivery further improves pharmacokinetic profiles, reduces systemic toxicity, and enhances targeting of intracellular infections and biofilm-associated communities. As research advances, composite and hybrid nanosystems have the potential to become a cornerstone in the clinical management of MDR infections.
To manage an infection, there is a need for an early and accurate diagnosis of a particular infection. Conventional culture-based detection methods are reliable but time-consuming and require more labor work. With the advancement of nanotechnology, the diagnostic methods have been revolutionized, as these techniques are considered to have point-of-care abilities making the diagnostic methods more reliable, fast, sensitive, and accurate.210 Nanodiagnostics have all the physicochemical attributes of the nanoparticles, like tunable magnetic behavior, high surface area to volume ratio, and unique optical properties to identify pathogens with more accuracy and instantly. The AuNP have surface plasmon resonance, and these particles are being used in calorimetric assays. The AuNPs are engineered and tagged with antibodies and DNA/RNA probs, they can identify the DNA and RNA containing pathogens and antigens by visible color alteration, reducing the need for fancy instrumentation.211 Multiplexed colorimetric and plasmonic biosensors based on AuNPs have been designed to rapidly detect multiple bacterial strains and distinguish between susceptible and resistant phenotypes. Functionalized AuNPs can bind specifically to target DNA or proteins such as the mecA gene of MRSA, enabling visual or electronic readouts for point-of-care diagnosis. Their versatility is demonstrated in the creation of lateral flow assays (LFAs) and surface-enhanced Raman scattering platforms that allow for multiplexed detection with high sensitivity and specificity.212,213
For the capturing of pathogens and biological specimen enrichment, magnetic nanoparticles (MNPs), are used. The particles are engineered to tag with ligands that interact with pathogenic cells, ensuring the separation of these pathogens by magnetic filtration before detection. These magnetic particles are integrated with biosensors to reduce the detection time. Such biosensor systems are used for the detection of Salmonella, M. tuberculosis, and E. coli in sputum and blood.214 In addition, MNPs, especially iron oxide-based particles, offer rapid and efficient separation of bacterial DNA/RNA from complex clinical samples. MNPs are conjugated with capture probes or antibodies, facilitating targeted extraction of nucleic acids encoding resistance factors such as New Delhi metallo-β-lactamase-1 (blaNDM-1) and vancomycin resistance (vanA). Integrated with PCR, isothermal amplification, or microfluidic devices, magnetic nanoparticle platforms achieve quick turnaround times and enable downstream identification of MDR bacteria, providing reliable tools for surveillance and outbreak containment.215–217 In multiplex pathogen detection, QDs are used, which are nanocrystal semiconductors and give fluorescence according to size. Their broad excitation spectra and higher photostability enable to detection of several pathogens simultaneously. It is usually used for the detection of hospital-based infections caused by MRSA and P. aeruginosa.218
Another diagnostic platform is Graphene-based field effect transistors (FETs). These sensors can detect the binding ligands with extraordinary sensitivity and in real-time. These are tagged with specific antibodies or aptamers, and they can identify RNA, bacterial toxins, and whole complete cells within a short time. It can detect a very low concentration of MRSA in wound specimens to 10 CFU/mL.219 Furthermore, Graphene, with its large surface area and high electron mobility, is engineered into transistor-based biosensors capable of detecting nucleic acids (such as mecA, blaNDM-1, vanA) at extremely low concentrations critical for early diagnosis and monitoring. These devices enable rapid, label-free identification of MDR bacteria and can be integrated into portable or wearable technologies for real-time clinical decision support.220,221
Silver nanoparticles have been incorporated into electrochemical and optical biosensors for the rapid detection of bacterial pathogens. For example, AgNP-modified electrodes enhance the sensitivity of DNA hybridization sensors that target resistance genes in E. coli and S. aureus. Silver’s strong plasmonic and antimicrobial properties also enable dual identification and viability assessment.222–229
Upconversion nanoparticles (UCNPs) add further versatility, providing dual-mode (luminescence and colorimetric) sensing capabilities. Conjugated with probes for resistance genes, UCNPs facilitate highly sensitive and instrument-free identification, which is essential for deployment in resource-limited settings.230–232 Likewise, CNTs, often integrated into nanocomposite sensors with metals, supply electronic biosensors with ultra-sensitive, label-free detection capacity. Their high conductivity and surface area allow rapid identification of microbial markers, directly from clinical samples.233,234
Finally, hybrid platforms that combine magnetic beads with functionalized nanoparticles streamline laboratory workflows by enabling automated sample enrichment, nucleic acid extraction, and detection of resistance genes. These magnetic nanoplatforms are especially valuable for high-throughput diagnostic pipelines, where speed and sample processing efficiency are critical.213,215,235 Collectively, these complementary nanoparticle technologies form the backbone of next-generation diagnostic systems for multidrug-resistant infections. Their integration into clinical microbiology and molecular diagnostics promises a future of faster, more accurate, and multiplexed identification, supporting improved patient outcomes and better infection control.
Another diagnostic application of nanoparticles is LFAs. This method is commonly employed in pregnancy tests, and further advancement in nanobiotechnology enables the detection of bacteria with the help of nanoparticles. For instance, pathogens like Streptococcus pneumoniae (S. pneumoniae) and Clostridium difficile can be easily detected from respiratory and stool samples by using AuNPs-based lateral flow assays.236 These assays are particularly useful in low-resource healthcare settings. Furthermore, there is a need to address challenges related to reproducibility, regulatory approval, and scalability, although these diagnostic methods are reliable, efficient, and rapidly diagnosed.
Furthermore, theranostic nanomaterials represent a major advance in the management of MDR infections, combining diagnostic and therapeutic functionalities within a single nanoplatform. These systems such as AuNPs, QDs, UCNPs, and multifunctional polymeric carriers are being engineered to simultaneously identify pathogens, monitor infection status, and administer targeted antimicrobial therapy. For example, gold nanorods conjugated with antibiotics have enabled rapid photoacoustic imaging of infection sites followed by heat-triggered drug release, while luminescent quantum-dot nanoassemblies facilitate both the swift detection of MDR bacteria and photodynamic microbial eradication. Lipid-based nano-formulations and magnetic nanoparticles can be functionalized for dual imaging and site-specific drug delivery, supporting real-time monitoring of treatment efficacy in vivo.237,238
Integration of diagnostic markers and therapeutics within theranostic nanoagents allows for precise infection targeting, dynamic assessment of therapeutic response, and minimized recurrence rates. These technologies increasingly incorporate stimuli-responsive functions such as light or pH-triggered drug release, providing tailored management of infections with complex resistance profiles. Recent reviews emphasize that rationally designed theranostic nanoplatforms can improve detection sensitivity, enhance pathogen eradication, and facilitate the translational progress of novel antimicrobial technologies against MDR bacteria and fungi. Collectively, theranostic strategies hold considerable promise for advancing personalized and precision medicine in infectious disease management.237,238
The AMR arises through complex bacterial adaptation mechanisms, principally including drug target modification, enzymatic degradation or inactivation of antimicrobial agents, active efflux of drugs by membrane transporters, decreased membrane permeability, and robust biofilm formation.239–241 These mechanisms are often triggered by repeated exposure to conventional antibiotics under conditions of strong selective pressure. Furthermore, bacteria frequently acquire and disseminate resistance genes through horizontal gene transfer mediated by plasmids, transposons, and bacteriophages, which accelerates the spread of resistance between different strains and even across species boundaries.242,243 Nanoparticles offer fundamentally different and advantageous approaches to overcoming AMR due to their unique nanoscale size, high surface area-to-volume ratio, and customizable surface properties.244,245 Unlike traditional antibiotics, which typically act via a single molecular target, nanomaterials exert multifactorial antibacterial actions: they can disrupt cell membranes physically, generate ROS that induce oxidative damage, interfere with bacterial metabolic pathways, suppress efflux pump activity, and penetrate biofilms that shield bacteria from conventional drugs.16,161,246–249 Metallic nanoparticles such as silver and copper oxide have demonstrated the ability to maintain potent activity against a broad spectrum of microbes, with minimal resistance emergence even after repeated exposure.250,251 Likewise, nanocarriers loaded with antimicrobial peptides or antibiotics enhance drug delivery to infection sites, improve pharmacokinetics, and allow for lower effective doses, further reducing the selective pressure that typically drives resistance.150,252,253
This multiplexed mechanism of action drastically diminishes the likelihood that bacteria will successfully evolve simultaneous resistance to all affected pathways, as opposed to the rapid adaptation observed with monotherapeutic agents. Nevertheless, cautious monitoring and innovation are essential, as rare cases of bacterial adaptation to specific nanomaterials have been reported, although at a much lower frequency than with traditional antibiotics.247,249 Overall, current evidence indicates that well-designed nanoparticles disrupt bacterial survival through multiple mechanisms, greatly impeding the development of lasting resistance. Sustained research and judicious clinical application will be crucial for maintaining efficacy against multidrug-resistant pathogens.
With an increase in antimicrobial-resistant strains, nanotechnology has aimed to develop better therapeutics. In the last decade, the research on nanoparticles showed progress from laboratory-based research to preclinical and clinical trials, which showed potential outcomes in the treatment of multidrug-resistant infections. This progress is a multidisciplinary effort by microbiology, material science, and the engineering of nanoparticles, pharmaceutical engineering is on the list for the development of multifunctional nanoparticles.254
Many formulations of the nanoparticles showed their efficacy by overcoming various resistant mechanisms, such as disruption of biofilm, penetration of the membrane, and inhibition of the efflux pump. The silver nanoparticles are used for the treatment of various multidrug-resistant infections caused by pathogens like E. coli, S. aureus, K. pneumoniae. These nanoparticles use a multifaceted mode of action, like DNA binding and oxidative stress, to reduce the bacterial capability to develop resistance. The silver nanoparticles are formulated for wound dressing and are also being evaluated for burn infections and diabetic foot ulcers.255
Lipid-based nanoparticles, liposomes, encapsulate less soluble antibiotics like rifampicin and vancomycin to improve penetration into the tissues and bioavailability. Different liposomal formulations, like Arikayce, are used for the treatment of Mycobacterium avium complex (MAC), and it is an FDA-approved nanoparticle for the treatment of pulmonary infections. These formulations have the potential to deliver existing drugs and enhance their therapeutic effects.256
The biodegradable polymeric fabricated material, like PLGA, has been employed for targeted drug delivery, reducing the development of resistance, and reducing toxicity. These nanoparticles showed promising results in animal models. The nanoparticles are used as carriers for dual-functional drugs, along with biofilm-disrupting material or quorum-sensing inhibitors. The transport of these dual-functional drugs is currently under study, particularly for chronic wound infections and catheter-linked infections.257
The magnetic nanoparticles use physical means along with antibiotics to kill bacteria and other pathogens. These hybrid particles can destroy biofilms, which is not easy to disrupt by conventional medicines, however, these magnetic nanoparticles have been studied only under laboratory conditions, and no pre-clinical or clinical trials have been conducted yet. However, these nanoparticles may play a role in the treatment of multidrug-resistant infections by theranostics shortly.258
The small interfering RNA-loaded nanoparticles are another emerging nano-vaccine against the immune system of multidrug-resistant pathogens. The preclinical and in vitro trials have been started in which these nanoparticles carry siRNA or host-specific antigens to directly target the bacterial or pathogenic virulence factors or the resistance genes, therefore directly reducing the pathogenic load.259 The nanoparticles for the treatment of multidrug infections, are under consideration, as listed in Table 2.
 |
Table 2 Clinical Trial Phase 1 of Nanoparticles for Treatment of Bacterial Infections
|
The challenges in clinical translation are manufacturing limitations, regulatory uncertainty, and safety concerns. The biocompatibility and safety of nanomaterials are the major concerns. The nanoparticles may aggregate in vital organs like spleen, kidneys, and liver, causing inflammation, immunogenicity, and oxidative stress. The long-term certainty about the toxicity of many nanoparticles is not available, and regulatory cautions are needed for clinical usage.260 Reproducibility and scalability are other hurdles. As the formulations of nanoparticles are reproducible in the Laboratory reproducing these results in industries is difficult. The standardization and quality control of these nanoparticles are needed. The regulatory guidelines for nanoparticles are not universal.261
The European Medicines Agency (EMA) and Food and Drug Administration (FDA) issued general guidelines, but they are adapted from conventional antibiotics. The specific guidelines for nanoparticle approval and usage are required. To develop nanoparticles and their formulations, a high cost is required, which discourages pharmaceutical companies is another hurdle.262
One of the major challenges in translating nanomedicine into clinical success is achieving efficient and selective delivery of nanoparticles to the intended site of infection or disease. Although nanoparticles are designed with the goal of targeted therapy, in practice, the majority of systemically administered nanoparticles undergo conventional biodistribution. Most particles are sequestered by the mononuclear phagocyte system, resulting in predominant accumulation within the liver, spleen, and other reticuloendothelial organs, rather than at the desired pathological site.21,22 This nonspecific distribution limits therapeutic efficacy and may increase the risk of off-target effects.
To address these limitations, two principal strategies for nanoparticle targeting have been developed: passive and active targeting. Passive targeting exploits the inherent physicochemical properties of nanoparticles, such as size and surface charge, to take advantage of physiological phenomena like the EPR effect. The EPR effect can facilitate accumulation in tumors or inflamed tissues by allowing nanoparticles to permeate and be retained in areas with leaky vasculature. However, this effect varies greatly between tissue types and is often less robust in infectious or inflamed regions compared to solid tumors, resulting in inconsistent delivery outcomes.155 Active targeting, in contrast, involves the functionalization of nanoparticle surfaces with specific ligands, such as antibodies, peptides, or small molecules, that bind to overexpressed or disease-specific receptors on target cells. While active targeting can enhance cellular uptake and specificity, it remains limited by numerous physiological barriers, including complex vascular architecture, extracellular matrices, variable blood flow, and immune clearance mechanisms. Additionally, variability in target receptor expression or downregulation in diseased tissues can further reduce targeting efficiency.23,263
Despite significant progress in nanoparticle engineering and targeting strategies, current evidence indicates that only a very small fraction often less than 1% of the systemically administered nanoparticle dose typically reaches the desired site of action.21,22 The majority accumulates in off-target organs, which underscores the need for continued research to overcome biological and physiological barriers, enhance nanoparticle stability in circulation, and further optimize both passive and active targeting techniques. Achieving these advances is critical for the clinical translation and success of nanomedicine-based therapies, particularly for the treatment of infectious diseases and multidrug-resistant infections.
To overcome these challenges, several approaches are employed, including the use of FDA-approved polymers to manufacture nanoparticles, and collaboration with industry, educational institutions, and other regulatory departments to harmonize guidelines. Moreover, clinical trials are encouraged to utilize these nanoparticles in real-world settings, making the approval of safe nanomaterials possible.264 The more clinical practice of this nanomaterial will also help to improve and specify the safety guidelines of these nanoparticles.
To understand the therapeutic potential of nanoparticles concerning antimicrobial resistance, different strategies have been used. Firstly, there is a need for nanoparticles to transition from in vitro studies to pre-clinical and clinical trials. For this purpose, translational studies are required for well-elaborated experimentation on animal models and then on early-phase human trials to evaluate the biosafety concerns, efficacy, and pharmacokinetics.265 Secondly, more research is required on the dual-functional nanoparticles, the particles that deliver antibiotics, as well as factors that disrupt biofilms, influence immune response and specifically target the infection site. Introducing various stimuli, specific ligands, and other drugs can probably enhance the efficacy of these nanoparticles.266 Thirdly, standardized protocols should be used to evaluate, characterize, and approve these nanoparticles; for this purpose, multidisciplinary collaborations of material scientists, microbiologists, pharmacists, and clinicians are encouraged. The combined efforts would contribute to the highly efficient innovation of these nanoparticles and also avoid redundant efforts. The open access to the data also promotes the rational design of more therapeutics and transparency.267
Moreover, different policy frameworks as well as funding agencies should play a role in scientific advancement, by providing funds, the commercialization of Nanomedicine could be made possible. By using these approaches, nanomedicine would play a role in treating several multidrug-resistant infections.268
Nanomedicine can greatly improve the fight against infections that resist multiple drugs by helping medicines better target problem areas, fighting biofilms, and preventing harsh side effects. Each nanomaterial adds a different way to solve microbial resistance. Nano-enabled testing helps identify infections early and correctly, which allows healthcare officers to begin the treatment sooner. Because antibiotics are used for both treatment and identification, we are seeing the emergence of theranostics in infectious diseases. Still, issues related to security, growth, and following rules remain a problem. To make progress, these problems must be investigated by thorough research, standardized approaches, and cooperation between different professions. Using the strengths of nanotechnology with artificial intelligence, precision medicine, and Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) technology has the potential to greatly impact antimicrobial approaches in the future. More funding for studies, supportive government policies, and teamwork between public and private organizations will help nanomedicine treat antimicrobial resistance and guide efforts in controlling infectious diseases.
Naveed Ahmed would like to acknowledge the Applied College, University of Tabuk for providing the facilities to conduct this informative review article.
Alqassem Hamdallah Abuarqoub is affiliated with Al-Wafi Group for Marketing and Int’l Trade Co. Ltd. The company had no role in the design, conduct, manuscript preparation, or decision to publish this review article. The authors report no other conflicts of interest in this work.
1. Liu C, Hong Q, Chang RYK, Kwok PCL, Chan H-K. Phage–antibiotic therapy as a promising strategy to combat multidrug-resistant infections and to enhance antimicrobial efficiency. Antibiotics. 2022;11(5):570. doi:10.3390/antibiotics11050570
2. De Oliveira DM, Forde BM, Kidd TJ, et al. Antimicrobial resistance in ESKAPE pathogens. Clinical Microbiology Reviews. 2020;33(3). doi:10.1128/cmr.00181-19
3. WHO. Antimicrobial resistance. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
4. Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309–318. doi:10.1179/2047773215y.0000000030
5. Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(1):1–12. doi:10.1086/595011
6. Pendleton JN, Gorman SP, Gilmore BF. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther. 2013;11(3):297–308. doi:10.1586/eri.13.12
7. Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–1098. doi:10.1016/s1473-3099(13)70318-9
8. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P t. 2015;40(4):277–283.
9. Wahab S, Salman A, Khan Z, Khan S, Krishnaraj C, Yun SI. Metallic Nanoparticles: a Promising Arsenal against Antimicrobial Resistance-Unraveling Mechanisms and Enhancing Medication Efficacy. Int J Mol Sci. 2023;24(19):14897. doi:10.3390/ijms241914897
10. Tang Z, Ma Q, Chen X, et al. Recent Advances and Challenges in Nanodelivery Systems for Antimicrobial Peptides (AMPs). Antibiotics (Basel). 2021;10(8). doi:10.3390/antibiotics10080990
11. Mandal S, Chakraborty S, Manna S, Mandal S. Antimicrobial nanoparticles: current landscape and future challenges. RSC Pharmaceut. 2024;1:388–402. doi:10.1039/D4PM00032C
12. Zou W, McAdorey A, Yan H, Chen W. Nanomedicine to Overcome Antimicrobial Resistance: challenges and Prospects. Nanomedicine. 2023;18(5):471–484. doi:10.2217/nnm-2023-0022
13. Song X, Liu P, Liu X, et al. Dealing with MDR bacteria and biofilm in the post-antibiotic era: application of antimicrobial peptides-based nano-formulation. Materials Science and Engineering. 2021;128:112318. doi:10.1016/j.msec.2021.112318
14. Hashem AH, Al Abboud MA, Alawlaqi MM, Abdelghany TM, Hasanin M. Synthesis of nanocapsules based on biosynthesized nickel nanoparticles and potato starch: antimicrobial, antioxidant, and anticancer activity. Starch‐Stärke. 2022;74(1–2):2100165. doi:10.1002/star.202100165
15. Hetta HF, Ramadan YN, Al-Harbi AI, et al. Nanotechnology as a promising approach to combat multidrug resistant bacteria: a comprehensive review and future perspectives. Biomedicines. 2023;11(2):413. doi:10.3390/biomedicines11020413
16. Solanki R, Makwana N, Kumar R, et al. Nanomedicines as a cutting-edge solution to combat antimicrobial resistance. RSC Adv. 2024;14(45):33568–33586. doi:10.1039/d4ra06117a
17. Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi:10.1016/j.addr.2012.09.037
18. Tekade RK, Kumar PV, Jain NK. Dendrimers in oncology: an expanding horizon. Chem Rev. 2009;109(1):49–87. doi:10.1021/cr068212n
19. Cruz A, Barbosa J, Antunes P, Bonifácio VDB, Pinto SN. A Glimpse into Dendrimers Integration in Cancer Imaging and Theranostics. International Journal of Molecular Sciences. 2023;24(6):5430. doi:10.3390/ijms24065430
20. Ameh T, Gibb M, Stevens D, Pradhan SH, Braswell E, Sayes CM. Silver and copper nanoparticles induce oxidative stress in bacteria and mammalian cells. Nanomaterials. 2022;12(14):2402. doi:10.3390/nano12142402
21. Wilhelm S, Tavares AJ, Dai Q, et al. Analysis of nanoparticle delivery to tumours. Nature Reviews Materials. 2016;1(5):16014. doi:10.1038/natrevmats.2016.14
22. Dai Q, Wilhelm S, Ding D, et al. Quantifying the Ligand-Coated Nanoparticle Delivery to Cancer Cells in Solid Tumors. ACS Nano. 2018;12(8):8423–8435. doi:10.1021/acsnano.8b03900
23. Anselmo AC, Mitragotri S. Nanoparticles in the clinic: an update. Bioeng Transl Med. 2019;4(3):e10143. doi:10.1002/btm2.10143
24. Koczula KM, Gallotta A. Lateral flow assays. Essays Biochem. 2016;60(1):111–120. doi:10.1042/ebc20150012
25. Lan L, Yao Y, Ping J, Ying Y. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens Bioelectron. 2017;91:504–514. doi:10.1016/j.bios.2017.01.007
26. Yamin D, Uskoković V, Wakil AM, et al. Current and Future Technologies for the Detection of Antibiotic-Resistant Bacteria. Diagnostics (Basel). 2023;13(20). doi:10.3390/diagnostics13203246
27. Singh A, Amiji MM. Application of nanotechnology in medical diagnosis and imaging. Current Opinion in Biotechnology. 2022;74:241–246. doi:10.1016/j.copbio.2021.12.011
28. Lowden O. How nanotechnology can benefit healthcare. Available from: https://blog.bccresearch.com/how-nanotechnology-can-benefit-healthcare-industry.
29. Uskoković V. Nanomedicine for the poor: a lost cause or an idea whose time has yet to come? Nanomedicine (Lond). Nanomedicine (London, England). 2021;16(14):1203–1218. doi:10.2217/nnm-2021-0024
30. Mitchell SL, Carlson EE. Tiny Things with Enormous Impact: nanotechnology in the Fight Against Infectious Disease. ACS Infect Dis. 2018;4(10):1432–1435. doi:10.1021/acsinfecdis.8b00138
31. Thwala LN, Ndlovu SC, Mpofu KT, Lugongolo MY, Mthunzi-Kufa P. Nanotechnology-Based Diagnostics for Diseases Prevalent in Developing Countries: current Advances in Point-of-Care Tests. Nanomaterials (Basel). 2023;13(7):1247. doi:10.3390/nano13071247
32. Wang C, Liu M, Wang Z, Li S, Deng Y, He N. Point-of-care diagnostics for infectious diseases: from methods to devices. Nano Today. 2021;37:101092. doi:10.1016/j.nantod.2021.101092
33. Lehnert T, Gijs MA. Microfluidic systems for infectious disease diagnostics. Lab on a Chip. 2024;24(5):1441–1493. doi:10.1039/d4lc00117f
34. Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST. Challenges and strategies in anti-cancer nanomedicine development: an industry perspective. Adv Drug Deliv Rev. 2017;108:25–38. doi:10.1016/j.addr.2016.04.025
35. Ventola CL. The nanomedicine revolution: part 2: current and future clinical applications. P t. 2012;37(10):582–591.
36. Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arabian Journal of Chemistry. 2019;12(7):908–931. doi:10.1016/j.arabjc.2017.05.011
37. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
38. Gregoriadis G, Ryman BE. Liposomes as carriers of enzymes or drugs: a new approach to the treatment of storage diseases. Biochem J. 1971;124(5):58p. doi:10.1042/bj1240058p
39. Barenholz Y. Doxil®–the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–134. doi:10.1016/j.jconrel.2012.03.020
40. Gregoriadis G. Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol. 1995;13(12):527–537. doi:10.1016/s0167-7799(00)89017-4
41. Chopra R, Blair S, Strang J, Cervi P, Patterson KG, Goldstone AH. Liposomal amphotericin B (AmBisome) in the treatment of fungal infections in neutropenic patients. J Antimicrob Chemother. 1991;28(93–104):93–104. doi:10.1093/jac/28.suppl_b.93
42. Schiffelers RM, Bakker-Woudenberg IAJM, Snijders SV, Storm G. Localization of sterically stabilized liposomes in Klebsiella pneumoniae-infected rat lung tissue: influence of liposome characteristics. Biochimica et Biophysica Acta (BBA) – Biomembranes. 1999;1421(2):329–339. doi:10.1016/S0005-2736(99)00139-X
43. Schiffelers RM, Storm G, ten Kate MT, Bakker-Woudenberg IA. Therapeutic efficacy of liposome-encapsulated gentamicin in rat Klebsiella pneumoniae pneumonia in relation to impaired host defense and low bacterial susceptibility to gentamicin. Antimicrob Agents Chemother. 2001;45(2):464–470. doi:10.1128/aac.45.2.464-470.2001
44. Liu P, Chen G, Zhang J. A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules. 2022;27(4):1372. doi:10.3390/molecules27041372
45. Liu Y, Bravo KMC, Liu J. Targeted liposomal drug delivery: a nanoscience and biophysical perspective. Nanoscale Horizons. 2021;6(2):78–94. doi:10.1039/D0NH00605J
46. Alfreahat I, Nsairat H, Aldeeb ID, Al-Samydai A, Alshaer W. In Vitro Potentiation of Doxorubicin Cytotoxicity Utilizing Clarithromycin Loaded-PEGylated Liposomes. Technology in Cancer Research & Treatment. 2025;24:15330338241312561. doi:10.1177/15330338241312561
47. Pisani S, Tufail S, Rosalia M, et al. Antibiotic-loaded nano-sized delivery systems: an insight into gentamicin and vancomycin. Journal of Functional Biomaterials. 2024;15(7):194. doi:10.3390/jfb15070194
48. Pulingam T, Foroozandeh P, Chuah J-A, Sudesh K. Exploring various techniques for the chemical and biological synthesis of polymeric nanoparticles. Nanomaterials. 2022;12(3):576. doi:10.3390/nano12030576
49. Ardean C, Davidescu CM, Nemeş NS, et al. Factors influencing the antibacterial activity of chitosan and chitosan modified by functionalization. International Journal of Molecular Sciences. 2021;22(14):7449. doi:10.3390/ijms22147449
50. Wathoni N, Herdiana Y, Suhandi C, Mohammed AFA, El-Rayyes A, Narsa AC. Chitosan/Alginate-Based Nanoparticles for Antibacterial Agents Delivery. Int J Nanomedicine. 2024;19:5021–5044. doi:10.2147/ijn.S469572
51. Al-Gethami W, Al-Qasmi N. Antimicrobial Activity of Ca-Alginate/Chitosan Nanocomposite Loaded with Camptothecin. Polymers. 2021;13(20):3559. doi:10.3390/polym13203559
52. Zielińska A, Carreiró F, Oliveira AM, et al. Polymeric Nanoparticles: production, Characterization, Toxicology and Ecotoxicology. Molecules. 2020;25(16):3731. doi:10.3390/molecules25163731
53. Satchanska G, Davidova S, Petrov PD. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers (Basel). 2024;16(8):1159. doi:10.3390/polym16081159
54. Gad S, Awd H, Ghorab M, Abdelwahab N, Nasr A. A recent review on polymeric nanoparticles: structure, Classification, and preparation methods. Records of Pharmaceutical and Biomedical Sciences. 2024;8(3):58–66. doi:10.21608/rpbs.2024.285214.1293
55. Yadav HKS, Almokdad AA, Shaluf SIM, Debe MS. Chapter 17 – Polymer-Based Nanomaterials for Drug-Delivery Carriers. In: Nanocarriers for Drug Delivery. Mohapatra SS, Ranjan S, Dasgupta N, Mishra RK, Thomas S. editors. Elsevier. 2019:531–556.
56. Geszke-Moritz M, Moritz M. Biodegradable Polymeric Nanoparticle-Based Drug Delivery Systems: comprehensive Overview, Perspectives and Challenges. Polymers (Basel). 2024;16(17):2536. doi:10.3390/polym16172536
57. Shao L, Shen S, Liu H. Recent advances in PLGA micro/nanoparticle delivery systems as novel therapeutic approach for drug-resistant tuberculosis. Frontiers in Bioengineering and Biotechnology. 2022;10:941077. doi:10.3389/fbioe.2022.941077
58. Petrilli R, Pinheiro DP, de Cássia Evangelista de Oliveira F, et al. Immunoconjugates for cancer targeting: a review of antibody-drug conjugates and antibody-functionalized nanoparticles. Current Medicinal Chemistry. 2021;28(13):2485–2520. doi:10.2174/0929867327666200525161359
59. Chandrakala V, Aruna V, Angajala G. Review on metal nanoparticles as nanocarriers: current challenges and perspectives in drug delivery systems. Emergent Materials. 2022;5(6):1593–1615. doi:10.1007/s42247-021-00335-x
60. Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev. 2012;41(7):2740–2779. doi:10.1039/c1cs15237h
61. Hegde M, Pai P, Shetty G, M KSB. Gold nanoparticle based biosensors for rapid pathogen detection: a Review. Environmental Nanotechnology, Monitoring & Management. 2022;18:100756. doi:10.1016/j.enmm.2022.100756
62. Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7(1):17–28. doi:10.1016/j.jare.2015.02.007
63. Raghupathi KR, Koodali RT, Manna AC. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir. 2011;27(7):4020–4028. doi:10.1021/la104825u
64. Sirelkhatim A, Mahmud S, Seeni A, et al. Review on Zinc Oxide Nanoparticles: antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015;7(3):219–242. doi:10.1007/s40820-015-0040-x
65. Mendes CR, Dilarri G, Forsan CF, et al. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci Rep. 2022;12(1):2658. doi:10.1038/s41598-022-06657-y
66. Azam A, Ahmed AS, Oves M, Khan MS, Habib SS, Memic A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomedicine. 2012;7:6003–6009. doi:10.2147/ijn.S35347
67. Ren G, Hu D, Cheng EW, Vargas-Reus MA, Reip P, Allaker RP. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int J Antimicrob Agents. 2009;33(6):587–590. doi:10.1016/j.ijantimicag.2008.12.004
68. Ahmed M, Muhsin ZA. Synergistic effect of gentamicin and iron oxide nanoparticles on phzM gene of Pseudomonas aeruginosa. Mikrobiolohichnyi Zhurnal. 2024;86(3):27–39. doi:10.15407/microbiolj86.03.027
69. Hammami I, Alabdallah NM, Jomaa AA, Kamoun M. Gold nanoparticles: synthesis properties and applications. Journal of King Saud University-Science. 2021;33(7):101560. doi:10.1016/j.jksus.2021.101560
70. Bansal SA, Kumar V, Karimi J, Singh AP, Kumar S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Advances. 2020;2(9):3764–3787. doi:10.1039/D0NA00472C
71. Anik MI, Mahmud N, Al Masud A, Hasan M. Gold nanoparticles (GNPs) in biomedical and clinical applications: a review. Nano Select. 2022;3(4):792–828. doi:10.1002/nano.202100255
72. Ahmad M, Saeed MU, Hafeez M, Cheema SM, Pervaiz J. Pulmonary Tuberculosis and COVID-19 Co-infections in Lahore, Pakistan: a retrospective cohort study: pulmonary Tuberculosis and COVID-19 Co-infections. Electronic. Journal of Medical Research. 2025;1(1):45–53.
73. Dykman LA, Khlebtsov NG. Gold nanoparticles in biology and medicine: recent advances and prospects. Acta Naturae. 2011;3(2):34–55. doi:10.32607/20758251-2011-3-2-34-55
74. Kus-Liśkiewicz M, Fickers P, Ben tahar I. Biocompatibility and Cytotoxicity of Gold Nanoparticles: recent Advances in Methodologies and Regulations. Int J Mol Sci. 2021;22(20):10952. doi:10.3390/ijms222010952
75. Yaqoob SB, Adnan R, Rameez Khan RM, Rashid M. Gold, Silver, and Palladium Nanoparticles: a Chemical Tool for Biomedical Applications. Review. Frontiers in Chemistry. 2020;8. doi:10.3389/fchem.2020.00376
76. Dey S, Mohanty D, Divya N, et al. A critical review on zinc oxide nanoparticles: synthesis, properties and biomedical applications. Intelligent Pharmacy. 2025;3(1):53–70. doi:10.1016/j.ipha.2024.08.004
77. Hussein SA, Taha GM, Moghazy MA. Comparative study on synthesis ZnO nanoparticles using green and chemical methods and its effect on crystallite size and optical properties. Aswan University Journal of Environmental Studies. 2023;4(4):205–218. doi:10.21608/aujes.2023.200916.1136
78. Hamouda R, Yousuf W, Mohammed AA, Darwish D, Abdeen E. Comparative Study Between Zinc Oxide Nanoparticles Synthesized By Chemical And Biological Methods In View Of Characteristics, Antibacterial Activity And Loading On Antibiotics In Vitro. Digest J Nanomater Biostruct. 2020;15(1):93–106.
79. Bhandari KP, Sapkota DR, Jamarkattel MK, Stillion Q, Collins RW. Zinc Oxide Nanoparticles-Solution-Based Synthesis and Characterizations. Nanomaterials (Basel). 2023;13(11):1795. doi:10.3390/nano13111795
80. Zhou X-Q, Hayat Z, Zhang -D-D, et al. Zinc oxide nanoparticles: synthesis, characterization, modification, and applications in food and agriculture. Processes. 2023;11(4):1193. doi:10.3390/pr11041193
81. Kim I, Viswanathan K, Kasi G, Thanakkasaranee S, Sadeghi K, Seo J. ZnO nanostructures in active antibacterial food packaging: preparation methods, antimicrobial mechanisms, safety issues, future prospects, and challenges. Food Reviews International. 2022;38(4):537–565. doi:10.1080/87559129.2020.1737709
82. Aref HG, Said AM, Ahmed EK, AbdelKader MM, Elkady AM, Mohamed SA. Biomedical Applications and Toxicological Aspects of Zinc Oxide Nanoparticles: a Review Article. Sohag Medical Journal. 2023;27(1):18–27. doi:10.21608/smj.2022.173745.1354
83. Egbuna C, Parmar VK, Jeevanandam J, et al. Toxicity of Nanoparticles in Biomedical Application: nanotoxicology. J Toxicol. 2021;2021:9954443. doi:10.1155/2021/9954443
84. Kim CS, Nguyen HD, Ignacio RM, et al. Immunotoxicity of zinc oxide nanoparticles with different size and electrostatic charge. Int J Nanomedicine. 2014;9(Suppl 2):195–205. doi:10.2147/ijn.S57935
85. Xu L, Wang YY, Huang J, Chen CY, Wang ZX, Xie H. Silver nanoparticles: synthesis, medical applications and biosafety. Theranostics. 2020;10(20):8996–9031. doi:10.7150/thno.45413
86. Sati A, Ranade TN, Mali SN, Ahmad Yasin HK, Pratap A. Silver Nanoparticles (AgNPs): comprehensive Insights into Bio/Synthesis, Key Influencing Factors, Multifaceted Applications, and Toxicity─A 2024 Update. ACS Omega. 2025;10(8):7549–7582. doi:10.1021/acsomega.4c11045
87. Meher A, Tandi A, Moharana S, et al. Silver nanoparticle for biomedical applications: a review. Hybrid Advances. 2024;6:100184. doi:10.1016/j.hybadv.2024.100184
88. Fahim M, Shahzaib A, Nishat N, Jahan A, Bhat TA, Inam A. Green synthesis of silver nanoparticles: a comprehensive review of methods, influencing factors, and applications. JCIS Open. 2024;16:100125. doi:10.1016/j.jciso.2024.100125
89. Croitoru G-A, Pîrvulescu D-C, Niculescu AG, Grumezescu AM, Antohi AM, Nicolae C-L. Metallic nanomaterials–targeted drug delivery approaches for improved bioavailability, reduced side toxicity, and enhanced patient outcomes. Romanian Journal of Morphology and Embryology. 2024;65(2):145. doi:10.47162/RJME.65.2.01
90. Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275(1):177–182. doi:10.1016/j.jcis.2004.02.012
91. Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346–2353. doi:10.1088/0957-4484/16/10/059
92. Zhang XF, Liu ZG, Shen W, Gurunathan S. Silver Nanoparticles: synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int J Mol Sci. 2016;17(9):1534. doi:10.3390/ijms17091534
93. Rodrigues AS, Batista JGS, Rodrigues MÁV, et al. Advances in silver nanoparticles: a comprehensive review on their potential as antimicrobial agents and their mechanisms of action elucidated by proteomics. Review. Frontiers in Microbiology. 2024;15. doi:10.3389/fmicb.2024.1440065
94. Edwards-Jones V. Silver nanoparticles: an overview of scientific toxicity and safety data and introduction of a new dressing, Venus Ag. Wounds UK. 2022;18(4):22–29.
95. Bruna T, Maldonado-Bravo F, Jara P, Caro N. Silver nanoparticles and their antibacterial applications. International Journal of Molecular Sciences. 2021;22(13):7202. doi:10.3390/ijms22137202
96. Khleifat K, Qaralleh H, Al-Limoun M. Antibacterial activity of silver nanoparticles synthesized by aspergillus flavusand its synergistic effect with antibiotics. J Pure Appl Microbiol. 2022;16(3):1722–1735. doi:10.22207/JPAM.16.3.13
97. Chis AA, Dobrea C, Morgovan C, et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules. 2020;25(17). doi:10.3390/molecules25173982
98. Svenson S, Tomalia DA. Dendrimers in biomedical applications—reflections on the field. Advanced Drug Delivery Reviews. 2012;64:102–115. doi:10.1016/j.addr.2012.09.030
99. Li X, Naeem A, Xiao S, Hu L, Zhang J, Zheng Q. Safety Challenges and Application Strategies for the Use of Dendrimers in Medicine. Pharmaceutics. 2022;14(6):1292. doi:10.3390/pharmaceutics14061292
100. Zamboni WC. 21 – Carrier-mediated and artificial-cell targeted cancer drug delivery. In: Prakash S, editor. Artificial Cells, Cell Engineering and Therapy. Woodhead Publishing; 2007:469–501.
101. Tomalia D, Fréchet J. Introduction to the dendritic state. Dendrimers and Other Dendritic Polymers. 2001;2001;1–44.
102. Tomalia DA. The dendritic state. Materials Today. 2005;8(3):34–46. doi:10.1016/S1369-7021(05)00746-7
103. Tomalia DA, Baker H, Dewald J, et al. A new class of polymers: starburst-dendritic macromolecules. Polymer Journal. 1985;17(1):117–132. doi:10.1295/polymj.17.117
104. Svenson S. Dendrimers as versatile platform in drug delivery applications. European Journal of Pharmaceutics and Biopharmaceutics. 2009;71(3):445–462. doi:10.1016/j.ejpb.2008.09.023
105. Malik N. Dendrimer Conjugates: Evaluation as Novel Anticancer Therapeutics. University of London, University College London (United Kingdom); 1999.
106. Fateh ST, Aghaii AH, Aminzade Z, et al. Inorganic nanoparticle-cored dendrimers for biomedical applications: a review. Heliyon. 2024;10(9):e29726. doi:10.1016/j.heliyon.2024.e29726
107. Li L, Deng Y, Zeng Y, et al. The application advances of dendrimers in biomedical field. VIEW. 2023;4(6):20230023. doi:10.1002/VIW.20230023
108. Gorain B, Pandey M, Choudhury H, Jain GK, Kesharwani P. Dendrimer for solubility enhancement. In: Dendrimer-based nanotherapeutics. Elsevier. 2021:273–283.
109. Fritea L, Banica F, Costea TO, et al. Metal nanoparticles and carbon-based nanomaterials for improved performances of electrochemical (Bio) sensors with biomedical applications. Materials. 2021;14(21):6319. doi:10.3390/ma14216319
110. Maleki Dizaj S, Mennati A, Jafari S, Khezri K, Adibkia K. Antimicrobial activity of carbon-based nanoparticles. Adv Pharm Bull. 2015;5(1):19–23. doi:10.5681/apb.2015.003
111. Azizi-Lalabadi M, Hashemi H, Feng J, Jafari SM. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Advances in Colloid and Interface Science. 2020;284:102250. doi:10.1016/j.cis.2020.102250
112. Jyothirmayee AV. The Trio of Carbon Nanomaterials: fullerene, CNT, and Graphene—A Review of Their Discovery and Development in the Last Decade. Premier J Sci. 2025;9:100075. doi:10.70389/PJS.100075
113. Du X, Zhang M, Ma Y, et al. Size-dependent antibacterial of carbon dots by selective absorption and differential oxidative stress of bacteria. Journal of Colloid and Interface Science. 2023;634:44–53. doi:10.1016/j.jcis.2022.12.025
114. Laganà A, Visalli G, Corpina F, Ferlazzo M, Di Pietro A, Facciolà A. Antibacterial activity of nanoparticles and nanomaterials: a possible weapon in the fight against healthcare-associated infections. Eur Rev Med Pharmacol Sci. 2023;27(8):3645–3663.
115. Kharlamova MV, Kramberger C. Cytotoxicity of Carbon Nanotubes, Graphene, Fullerenes, and Dots. Nanomaterials. 2023;13(9):1458. doi:10.3390/nano13091458
116. Liu S, Zeng TH, Hofmann M, et al. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: membrane and Oxidative Stress. ACS Nano. 2011;5(9):6971–6980. doi:10.1021/nn202451x
117. Kang S, Herzberg M, Rodrigues DF, Elimelech M. Antibacterial effects of carbon nanotubes: size does matter! Langmuir. 2008;24(13):6409–6413. doi:10.1021/la800951v
118. Amine Zarouki M, Hejji L, Azzouz A, Yaeh A, Cobo AJM, Kailasa SK. Carbon nanostructures with antibacterial and wound healing activities: recent progress and challenges. J Mater Chem B. 2025;13:9745–9803. doi:10.1039/D5TB00272A
119. Raul PK, Thakuria A, Das B, et al. Carbon nanostructures as antibacterials and active food-packaging materials: a review. ACS omega. 2022;7(14):11555–11559. doi:10.1021/acsomega.2c00848
120. Mohammed H, Kumar A, Bekyarova E, et al. Antimicrobial Mechanisms and Effectiveness of Graphene and Graphene-Functionalized Biomaterials. A Scope Review. Front Bioeng Biotechnol. 2020;8:465. doi:10.3389/fbioe.2020.00465
121. Tene T, Bellucci S, Pachacama J, et al. Synthesis of metal nanoparticles on graphene oxide and antibacterial properties. Original Research. Frontiers in Chemistry. 2024;12. doi:10.3389/fchem.2024.1426179
122. Du T, Huang B, Cao J, et al. Ni nanocrystals supported on graphene oxide: antibacterial agents for synergistic treatment of bacterial infections. ACS omega. 2022;7(22):18339–18349. doi:10.1021/acsomega.2c00508
123. Ravikumar V, Mijakovic I, Pandit S. Antimicrobial Activity of Graphene Oxide Contributes to Alteration of Key Stress-Related and Membrane Bound Proteins. Int J Nanomedicine. 2022;17:6707–6721. doi:10.2147/ijn.S387590
124. Shankar K, Agarwal S, Mishra S, Bhatnagar P, Siddiqui S, Abrar I. A review on antimicrobial mechanism and applications of graphene-based materials. Biomaterials Advances. 2023;150:213440. doi:10.1016/j.bioadv.2023.213440
125. Itoo AM, Vemula SL, Gupta MT, et al. Multifunctional graphene oxide nanoparticles for drug delivery in cancer. Journal of Controlled Release. 2022;350:26–59. doi:10.1016/j.jconrel.2022.08.011
126. Karaky N. The Use of Metal Ions and Graphene-Based Compounds as Novel Antimicrobials Against Multidrug Resistant Pseudomonas Aeruginosa, Klebsiella Pneumoniae and Staphylococcus Aureus. Manchester Metropolitan University; 2020.
127. Saleemi MA, Kong YL, Yong PVC, Wong EH. An Overview of Antimicrobial Properties of Carbon Nanotubes-Based Nanocomposites. Adv Pharm Bull. 2022;12(3):449–465. doi:10.34172/apb.2022.049
128. Vardharajula S, Ali SZ, Tiwari PM, et al. Functionalized carbon nanotubes: biomedical applications. Int J Nanomedicine. 2012;7:5361–5374. doi:10.2147/ijn.S35832
129. Mohammadi E, Zeinali M, Mohammadi-Sardoo M, Iranpour M, Behnam B, Mandegary A. The effects of functionalization of carbon nanotubes on toxicological parameters in mice. Human & Experimental Toxicology. 2020;39(9):1147–1167. doi:10.1177/0960327119899988
130. Mousavi SR, Estaji S, Kiaei H, et al. A review of electrical and thermal conductivities of epoxy resin systems reinforced with carbon nanotubes and graphene-based nanoparticles. Polymer Testing. 2022;112:107645. doi:10.1016/j.polymertesting.2022.107645
131. Teixeira-Santos R, Gomes M, Gomes LC, Mergulhão FJ. Antimicrobial and anti-adhesive properties of carbon nanotube-based surfaces for medical applications: a systematic review. iScience. 2021;24(1):102001. doi:10.1016/j.isci.2020.102001
132. Liu D, Mao Y, Ding L. Carbon nanotubes as antimicrobial agents for water disinfection and pathogen control. Journal of Water and Health. 2018;16(2):171–180. doi:10.2166/wh.2018.228
133. Abo-Neima SE, Motaweh HA, Elsehly EM. Antimicrobial activity of functionalised carbon nanotubes against pathogenic microorganisms. IET Nanobiotechnology. 2020;14(6):457–464. doi:10.1049/iet-nbt.2019.0342
134. Hassani M, Tahghighi A, Rohani M, Hekmati M, Ahmadian M, Ahmadvand H. Robust antibacterial activity of functionalized carbon nanotube- levofloxacine conjugate based on in vitro and in vivo studies. Scientific Reports. 2022;12(1):10064. doi:10.1038/s41598-022-14206-w
135. Hou W, Shi G, Wu S, et al. Application of Fullerenes as Photosensitizers for Antimicrobial Photodynamic Inactivation: a Review. Front Microbiol. 2022;13:957698. doi:10.3389/fmicb.2022.957698
136. Bolshakova O, Lebedev V, Mikhailova E, et al. Fullerenes on a Nanodiamond Platform Demonstrate Antibacterial Activity with Low Cytotoxicity. Pharmaceutics. 2023;15(7):1984. doi:10.3390/pharmaceutics15071984
137. Nimibofa A, Newton EA, Cyprain AY, Donbebe W. Fullerenes: synthesis and applications. J Mater Sci. 2018;7(3):22.
138. Heredia DA, Durantini AM, Durantini JE, Durantini EN. Fullerene C60 derivatives as antimicrobial photodynamic agents. Journal of Photochemistry and Photobiology C. 2022;51:100471. doi:10.1016/j.jphotochemrev.2021.100471
139. Cheng J, Dorner-Reisel A, Wang T, et al. Biological characterization of the osteogenic, antibacterial and anti-inflammatory properties of fullerene-modified zirconia and titanium alloy implant surfaces. Diamond and Related Materials. 2025;155:112339. doi:10.1016/j.diamond.2025.112339
140. Javed Ansari M, Soltani A, Ramezanitaghartapeh M, et al. Improved antibacterial activity of sulfasalazine loaded fullerene derivative: computational and experimental studies. Journal of Molecular Liquids. 2022;348:118083. doi:10.1016/j.molliq.2021.118083
141. Ibrahim AA, Khan T, Nowlin K, et al. A rapid one-step synthesis of silver and copper coordinated chlorine functionalized fullerene nanoparticles with enhanced antibacterial activity. Nanoscale Advances. 2024;6(23):5833–5852. doi:10.1039/D4NA00732H
142. Ye L, Kollie L, Liu X, et al. Antitumor activity and potential mechanism of novel fullerene derivative nanoparticles. Molecules. 2021;26(11):3252. doi:10.3390/molecules26113252
143. Shariati A, Chegini Z, Ghaznavi-Rad E, Zare EN, Hosseini SM. PLGA-Based Nanoplatforms in Drug Delivery for Inhibition and Destruction of Microbial Biofilm. Front Cell Infect Microbiol. 2022;12:926363. doi:10.3389/fcimb.2022.926363
144. Verlee A, Mincke S, Stevens CV. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydrate Polymers. 2017;164:268–283. doi:10.1016/j.carbpol.2017.02.001
145. Aflakian F, Mirzavi F, Aiyelabegan HT, et al. Nanoparticles-based therapeutics for the management of bacterial infections: a special emphasis on FDA approved products and clinical trials. European Journal of Pharmaceutical Sciences. 2023;188:106515. doi:10.1016/j.ejps.2023.106515
146. Makabenta JMV, Nabawy A, Li CH, Schmidt-Malan S, Patel R, Rotello VM. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat Rev Microbiol. 2021;19(1):23–36. doi:10.1038/s41579-020-0420-1
147. Mba IE, Nweze EI. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: research progress, challenges, and prospects. World J Microbiol Biotechnol. 2021;37(6):108. doi:10.1007/s11274-021-03070-x
148. Gunasekaran T, Nigusse T, Dhanaraju MD. Silver nanoparticles as real topical bullets for wound healing. J Am Coll Clin Wound Spec. 2011;3(4):82–96. doi:10.1016/j.jcws.2012.05.001
149. Castillo RR, Vallet-Regí M. Recent advances toward the use of mesoporous silica nanoparticles for the treatment of bacterial infections. International Journal of Nanomedicine. 2021;Volume 16:4409–4430. doi:10.2147/IJN.S273064
150. Gao W, Chen Y, Zhang Y, Zhang Q, Zhang L. Nanoparticle-based local antimicrobial drug delivery. Adv Drug Deliv Rev. 2018;127:46–57. doi:10.1016/j.addr.2017.09.015
151. Rai M, Deshmukh SD, Ingle AP, Gupta IR, Galdiero M, Galdiero S. Metal nanoparticles: the protective nanoshield against virus infection. Crit Rev Microbiol. 2016;42(1):46–56. doi:10.3109/1040841x.2013.879849
152. Tapfumaneyi P, Imran M, Mohammed Y, Roberts MS. Recent advances and future prospective of topical and transdermal delivery systems. Mini Review. Frontiers in Drug Delivery. 2022;2. doi:10.3389/fddev.2022.957732
153. Teo MZY, Loo HL, Goh BH, Chuah LH. Progress in topical nanoformulations against bacterial skin and soft tissue infections- current trends. Drug Deliv Transl Res. 2025. doi:10.1007/s13346-025-01924-7
154. MubarakAli D, Saravanakumar K, Ganeshalingam A, et al. Recent Progress in Multifunctional Stimuli-Responsive Combinational Drug Delivery Systems for the Treatment of Biofilm-Forming Bacterial Infections. Pharmaceutics. 2024;16(8):976. doi:10.3390/pharmaceutics16080976
155. Danhier F. To exploit the tumor microenvironment: since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release. 2016;244(Pt A):108–121. doi:10.1016/j.jconrel.2016.11.015
156. Mout R, Moyano DF, Rana S, Rotello VM. Surface functionalization of nanoparticles for nanomedicine. Chemical Society Reviews. 2012;41(7):2539–2544. doi:10.1039/c2cs15294k
157. Baptista PV, McCusker MP, Carvalho A, et al. Nano-Strategies to Fight Multidrug Resistant Bacteria- “A Battle of the Titans”. Front Microbiol. 2018;9:1441. doi:10.3389/fmicb.2018.01441
158. Bharadwaj A, Rastogi A, Pandey S, Gupta S, Sohal JS. Multidrug-Resistant Bacteria: their Mechanism of Action and Prophylaxis. Biomed Res Int. 2022;2022(1):5419874. doi:10.1155/2022/5419874
159. Luceri A, Francese R, Lembo D, Ferraris M, Balagna C. Silver nanoparticles: review of antiviral properties, mechanism of action and applications. Microorganisms. 2023;11(3):629. doi:10.3390/microorganisms11030629
160. Modi SK, Gaur S, Sengupta M, Singh MS. Mechanistic insights into nanoparticle surface-bacterial membrane interactions in overcoming antibiotic resistance. Review. Frontiers in Microbiology. 2023;14:1135579. doi:10.3389/fmicb.2023.1135579
161. Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. International Journal of Nanomedicine. 2017;Volume 12:1227–1249. doi:10.2147/IJN.S121956
162. Xie W, Zhang S, Pan F, et al. Nanomaterial-based ROS-mediated strategies for combating bacteria and biofilms. Journal of Materials Research. 2021;36(4):822–845. doi:10.1557/s43578-021-00134-4
163. Mammari N, Lamouroux E, Boudier A, Duval RE. Current Knowledge on the Oxidative-Stress-Mediated Antimicrobial Properties of Metal-Based Nanoparticles. Microorganisms. 2022;10(2):437. doi:10.3390/microorganisms10020437
164. Barani M, Zeeshan M, Kalantar-Neyestanaki D, et al. Nanomaterials in the management of gram-negative bacterial infections. Nanomaterials. 2021;11(10):2535. doi:10.3390/nano11102535
165. Valenti GE, Alfei S, Caviglia D, Domenicotti C, Marengo B. Antimicrobial Peptides and Cationic Nanoparticles: a Broad-Spectrum Weapon to Fight Multi-Drug Resistance Not Only in Bacteria. Int J Mol Sci. 2022;23(11):6108. doi:10.3390/ijms23116108
166. Tamara FR, Lin C, Mi FL, Ho YC. Antibacterial Effects of Chitosan/Cationic Peptide Nanoparticles. Nanomaterials (Basel). 2018;8(2):88. doi:10.3390/nano8020088
167. Yan D, Li Y, Liu Y, Li N, Zhang X, Yan C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules. 2021;26(23):7136. doi:10.3390/molecules26237136
168. Huma ZE, Gupta A, Javed I, et al. Cationic Silver Nanoclusters as Potent Antimicrobials against Multidrug-Resistant Bacteria. ACS Omega. 2018;3(12):16721–16727. doi:10.1021/acsomega.8b02438
169. Tiwari AK, Gupta MK, Narayan RJ, Pandey PC. A whole cell fluorescence quenching-based approach for the investigation of polyethyleneimine functionalized silver nanoparticles interaction with Candida albicans. Original Research. Frontiers in Microbiology. 2023;14:1131122. doi:10.3389/fmicb.2023.1131122
170. Liu Z, Wang Y, Zu Y, et al. Synthesis of polyethylenimine (PEI) functionalized silver nanoparticles by a hydrothermal method and their antibacterial activity study. Materials Science and Engineering. 2014;42:31–37. doi:10.1016/j.msec.2014.05.007
171. Baselga M, Uranga-Murillo I, de Miguel D, et al. Silver Nanoparticles-Polyethyleneimine-Based Coatings with Antiviral Activity against SARS-CoV-2: a New Method to Functionalize Filtration Media. Materials. 2022;15(14):4742. doi:10.3390/ma15144742
172. Niu J, Tang G, Tang J, et al. Functionalized Silver Nanocapsules with Improved Antibacterial Activity Using Silica Shells Modified with Quaternary Ammonium Polyethyleneimine as a Bacterial Cell-Targeting Agent. Journal of Agricultural and Food Chemistry. 2021;69(23):6485–6494. doi:10.1021/acs.jafc.1c01930
173. Al-Maliki RM, Alsalhy QF, Al-Jubouri S, et al. Classification of nanomaterials and the effect of graphene oxide (GO) and recently developed nanoparticles on the ultrafiltration membrane and their applications: a review. Membranes. 2022;12(11):1043. doi:10.3390/membranes12111043
174. Jasim HK, Al-Ridah ZA, Naje AS. Graphene oxide–carbon nanotube composite membrane for enhanced removal of organic pollutants by forward osmosis. Desalination and Water Treatment. 2024;318:100363. doi:10.1016/j.dwt.2024.100363
175. Romero-Vargas Castrillón S, Perreault F, de Faria AF, Elimelech M. Interaction of Graphene Oxide with Bacterial Cell Membranes: insights from Force Spectroscopy. Environmental Science & Technology Letters. 2015;2(4):112–117. doi:10.1021/acs.estlett.5b00066
176. Chen KL, Bothun GD. Nanoparticles Meet Cell Membranes: probing Nonspecific Interactions using Model Membranes. Environmental Science & Technology. 2014;48(2):873–880. doi:10.1021/es403864v
177. Mondal SK, Chakraborty S, Manna S, Mandal SM. Antimicrobial nanoparticles: current landscape and future challenges. RSC Pharmaceutics. 2024;1(3):388–402. doi:10.1039/D4PM00032C
178. Ayanda OS, Mmuoegbulam AO, Okezie O, et al. Recent progress in carbon-based nanomaterials: critical review. Journal of Nanoparticle Research. 2024;26(5):106. doi:10.1007/s11051-024-06006-2
179. Sachdev S, Ansari SA, Ansari MI. Reactive oxygen species (ROS): an introduction. In: Reactive Oxygen Species in Plants: The Right Balance. Springer; 2023:1–22.
180. Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. International Journal of Molecular Sciences. 2021;22(9):4642. doi:10.3390/ijms22094642
181. Ekram B, Tolba E, El-Sayed AF, et al. Cell migration, DNA fragmentation and antibacterial properties of novel silver doped calcium polyphosphate nanoparticles. Scientific Reports. 2024;14(1):565. doi:10.1038/s41598-023-50849-z
182. Spirescu VA, Chircov C, Grumezescu AM, Andronescu E. Polymeric Nanoparticles for Antimicrobial Therapies: an Up-To-Date Overview. Polymers (Basel). 2021;13(5):724. doi:10.3390/polym13050724
183. Hada V, Chaturvedi K, Singhwane A, et al. Nanoantibiotic effect of carbon-based nanocomposites: epicentric on graphene, carbon nanotubes and fullerene composites: a review. 3 Biotech. 2023;13(5):147. doi:10.1007/s13205-023-03552-9
184. Madni A, Rehman S, Sultan H, et al. Mechanistic approaches of internalization, subcellular trafficking, and cytotoxicity of nanoparticles for targeting the small intestine. AAPS PharmSciTech. 2021;22:1–17.
185. Porzani SJ, Lorenzi AS, Eghtedari M, Nowruzi B. Interaction of dehydrogenase enzymes with nanoparticles in industrial and medical applications, and the associated challenges: a mini-review. Mini Reviews in Medicinal Chemistry. 2021;21(11):1351–1366. doi:10.2174/1570193X17666201119152944
186. Qu R, Chen M, Liu J, Xie Q, Liu N, Ge F. Blockage of ATPase-mediated energy supply inducing metabolic disturbances in algal cells under silver nanoparticles stress. Journal of Environmental Sciences. 2023;131:141–150. doi:10.1016/j.jes.2022.10.029
187. Liu J, Huang Z, Yin S, Zhou X, Jiang Y, Shao L. The lysosome-mitochondrion crosstalk engaged in silver nanoparticles-disturbed mitochondrial homeostasis. Science of the Total Environment. 2023;889:164078. doi:10.1016/j.scitotenv.2023.164078
188. Pandey RP, Mukherjee R, Priyadarshini A, et al. Potential of nanoparticles encapsulated drugs for possible inhibition of the antimicrobial resistance development. Biomedicine & Pharmacotherapy. 2021;141:111943. doi:10.1016/j.biopha.2021.111943
189. Joseph X, Akhil V, Arathi A, Mohanan P. Nanobiomaterials in support of drug delivery related issues. Materials Science and Engineering: B. 2022;279:115680. doi:10.1016/j.mseb.2022.115680
190. Sebak AA, El-Shenawy BM, El-Safy S, El-Shazly M. From passive targeting to personalized nanomedicine: multidimensional insights on nanoparticles’ interaction with the tumor microenvironment. Current Pharmaceutical Biotechnology. 2021;22(11):1444–1465. doi:10.2174/1389201021666201211103856
191. Mirghani R, Saba T, Khaliq H, et al. Biofilms: formation, drug resistance and alternatives to conventional approaches. AIMS Microbiology. 2022;8(3):239. doi:10.3934/microbiol.2022019
192. Lahiri D, Nag M, Sheikh HI, et al. Microbiologically-synthesized nanoparticles and their role in silencing the biofilm signaling cascade. Frontiers in Microbiology. 2021;12:636588. doi:10.3389/fmicb.2021.636588
193. Afrasiabi S, Partoazar A. Targeting bacterial biofilm-related genes with nanoparticle-based strategies. Frontiers in Microbiology. 2024;15:1387114. doi:10.3389/fmicb.2024.1387114
194. Federico S, Catania V, Palumbo FS, et al. Photothermal nanofibrillar membrane based on hyaluronic acid and graphene oxide to treat Staphylococcus aureus and Pseudomonas aeruginosa infected wounds. International Journal of Biological Macromolecules. 2022;214:470–479. doi:10.1016/j.ijbiomac.2022.06.144
195. Agreles MAA, Cavalcanti IDL, Cavalcanti IMF. Synergism between metallic nanoparticles and antibiotics. Applied Microbiology and Biotechnology. 2022;106(11):3973–3984. doi:10.1007/s00253-022-12001-1
196. Hulme J. Application of nanomaterials in the prevention, detection, and treatment of methicillin-resistant Staphylococcus aureus (MRSA). Pharmaceutics. 2022;14(4):805. doi:10.3390/pharmaceutics14040805
197. Nair A, Mallya R, Suvarna V, Khan TA, Momin M, Omri A. Nanoparticles—Attractive carriers of antimicrobial essential oils. Antibiotics. 2022;11(1):108. doi:10.3390/antibiotics11010108
198. Mercan D-A, Niculescu A-G, Grumezescu AM. Nanoparticles for antimicrobial agents delivery—an up-to-date review. International Journal of Molecular Sciences. 2022;23(22):13862. doi:10.3390/ijms232213862
199. Mektrirat R, Paengjun N, Chongrattanameteekul P, et al. Utilizing liposomal encapsulation approach to address nephrotoxic challenges of colistimethate sodium through a preclinical study. Frontiers in Pharmacology. 2023;14:1282464. doi:10.3389/fphar.2023.1282464
200. Asl FD, Mousazadeh M, Taji S, et al. Nano drug-delivery systems for management of AIDS: liposomes, dendrimers, gold and silver nanoparticles. Nanomedicine. 2023;18(3):279–302. doi:10.2217/nnm-2022-0248
201. Alshawwa SZ, Kassem AA, Farid RM, Mostafa SK, Labib GS. Nanocarrier drug delivery systems: characterization, limitations, future perspectives and implementation of artificial intelligence. Pharmaceutics. 2022;14(4):883. doi:10.3390/pharmaceutics14040883
202. Ozdal M, Gurkok S. Recent advances in nanoparticles as antibacterial agent. Admet dmpk. 2022;10(2):115–129. doi:10.5599/admet.1172
203. Ranpariya B, Salunke G, Karmakar S, et al. Antimicrobial Synergy of Silver-Platinum Nanohybrids With Antibiotics. Front Microbiol. 2020;11:610968. doi:10.3389/fmicb.2020.610968
204. Zhang J, Liu M, Guo H, et al. Nanotechnology-driven strategies to enhance the treatment of drug-resistant bacterial infections. WIREs Nanomedicine and Nanobiotechnology. 2024;16(3):e1968. doi:10.1002/wnan.1968
205. Shabatina TI, Vernaya OI, Melnikov MY. Hybrid Nanosystems of Antibiotics with Metal Nanoparticles-Novel Antibacterial Agents. Molecules. 2023;28(4):1603. doi:10.3390/molecules28041603
206. Ezeh CK, Dibua MEU. Anti-biofilm, drug delivery and cytotoxicity properties of dendrimers. Admet dmpk. 2024;12(2):239–267. doi:10.5599/admet.1917
207. Ali AA, Al Bostami RD, Al-Othman A. Nanogel-based composites for bacterial antibiofilm activity: advances, challenges, and prospects. RSC Adv. 2024;14(15):10546–10559. doi:10.1039/d4ra00410h
208. Yapa P, Munaweera I, Weerasekera MM, Weerasinghe L. Synergistic antimicrobial nanofiber membranes based on metal incorporated silica nanoparticles as advanced antimicrobial layers. RSC Advances. 2024;14(46):33919–33940. doi:10.1039/D4RA05052E
209. Parvin N, Joo SW, Mandal TK. Nanomaterial-Based Strategies to Combat Antibiotic Resistance: mechanisms and Applications. Antibiotics (Basel). 2025;14(2). doi:10.3390/antibiotics14020207
210. Ma Y, Song M, Li L, Lao X, Wong MC, Hao J. Advances in upconversion luminescence nanomaterial‐based biosensor for virus diagnosis. Exploration. 2022;2:20210216.
211. Ferrari E. Gold nanoparticle-based plasmonic biosensors. Biosensors. 2023;13(3):411. doi:10.3390/bios13030411
212. Yang J, Wang X, Sun Y, et al. Recent Advances in Colorimetric Sensors Based on Gold Nanoparticles for Pathogen Detection. Biosensors. 2022;13(1):29. doi:10.3390/bios13010029
213. Gutiérrez-Santana JC, Rosas-Espinosa V, Martinez E, Casiano-García E, Coria-Jiménez VR. Metal Nanoparticle-Based Biosensors for the Early Diagnosis of Infectious Diseases Caused by ESKAPE Pathogens in the Fight against the Antimicrobial-Resistance Crisis. Biosensors. 2024;14(7):339. doi:10.3390/bios14070339
214. Syed S, Rahaman A, Mondal A, Shaligram S, Pawar SP. Diagnosis of infectious diseases: complexity to convenience. Sensors & Diagnostics. 2024;3(3):354–380. doi:10.1039/D3SD00236E
215. Wang M, Jin L, Hang-Mei Leung P, et al. Advancements in magnetic nanoparticle-based biosensors for point-of-care testing. Front Bioeng Biotechnol. 2024;12:1393789. doi:10.3389/fbioe.2024.1393789
216. Chung H, Castro C, Im H, Lee H, Weissleder R. A magneto-DNA nanoparticle system for rapid detection and phenotyping of bacteria. Nature Nanotechnology. 2013;8(5):369–375. doi:10.1038/nnano.2013.70
217. Ma C, Li C, Wang F, et al. Magnetic Nanoparticles-Based Extraction and Verification of Nucleic Acids from Different Sources. Journal of Biomedical Nanotechnology. 2013;9(4):703–709. doi:10.1166/jbn.2013.1566
218. Saxena S, Punjabi K, Ahamad N, Singh S, Bendale P, Banerjee R. Nanotechnology approaches for rapid detection and theranostics of antimicrobial resistant bacterial infections. ACS Biomaterials Science & Engineering. 2022;8(6):2232–2257. doi:10.1021/acsbiomaterials.1c01516
219. Pandit S, Li M, Chen Y, et al. Graphene-based sensor for detection of bacterial pathogens. Sensors. 2021;21(23):8085. doi:10.3390/s21238085
220. Ding R, Chen Y, Wang Q, et al. Recent advances in quantum dots-based biosensors for antibiotics detection. J Pharm Anal. 2022;12(3):355–364. doi:10.1016/j.jpha.2021.08.002
221. Li C, Sun F. Graphene-Assisted Sensor for Rapid Detection of Antibiotic Resistance in Escherichia coli. Original Research. Frontiers in Chemistry. 2021;9:696906. doi:10.3389/fchem.2021.696906
222. Bashir S, Nawaz H, Majeed MI, et al. Rapid and sensitive discrimination among carbapenem resistant and susceptible E. coli strains using Surface Enhanced Raman Spectroscopy combined with chemometric tools. Photodiagnosis Photodyn Ther. 2021;34:102280. doi:10.1016/j.pdpdt.2021.102280
223. Madhu S, Ramasamy S, Choi J. Recent Developments in Electrochemical Sensors for the Detection of Antibiotic-Resistant Bacteria. Pharmaceuticals. 2022;15(12):1488. doi:10.3390/ph15121488
224. Zhang Y, Zhang K, Ma H. Electrochemical DNA biosensor based on silver nanoparticles/poly(3-(3-pyridyl) acrylic acid)/carbon nanotubes modified electrode. Anal Biochem. 2009;387(1):13–19. doi:10.1016/j.ab.2008.10.043
225. Cai H, Xu Y, Zhu N, He P, Fang Y. An electrochemical DNA hybridization detection assay based on a silver nanoparticle label. Analyst. 2002;127(6):803–808. doi:10.1039/b200555g
226. Gahlaut SK, Pathak A, Gupta BD. Recent Advances in Silver Nanostructured Substrates for Plasmonic Sensors. Biosensors. 2022;12(9):713. doi:10.3390/bios12090713
227. Imran M, Ehrhardt C, Bertino M, Raza M, Yadavalli V. Chitosan Stabilized Silver Nanoparticles for the Electrochemical Detection of Lipopolysaccharide: a Facile Biosensing Approach for Gram-Negative Bacteria. Micromachines. 2020;11(4):1–9. doi:10.3390/mi11040413
228. Hannah S, Dobrea A, Lasserre P, et al. Development of a Rapid, Antimicrobial Susceptibility Test for E. coli Based on Low-Cost, Screen-Printed Electrodes. Biosensors. 2020;10(11):153. doi:10.3390/bios10110153
229. Dabhade AH, Verma RP, Paramasivan B, Kumawat A, Saha B. Development of silver nanoparticles and aptamer conjugated biosensor for rapid detection of E. coli in a water sample. 3 Biotech. 2023;13(7):244. doi:10.1007/s13205-023-03663-3
230. Wu J, Wu J, Wei W, Zhang Y, Chen Q. Upconversion Nanoparticles Based Sensing: from Design to Point-of-Care Testing. Small. 2024;20(29):2311729. doi:10.1002/smll.202311729
231. Singh R, Dumlupinar G, Andersson-Engels S, Melgar S. Emerging applications of upconverting nanoparticles in intestinal infection and colorectal cancer. Int J Nanomedicine. 2019;14:1027–1038. doi:10.2147/ijn.S188887
232. Lv H, Liu J, Wang Y, et al. Upconversion nanoparticles and its based photodynamic therapy for antibacterial applications: a state-of-the-art review. Perspective. Frontiers in Chemistry. 2022;10:996264. doi:10.3389/fchem.2022.996264
233. Zor E, Mollarasouli F, Karadurmus L, Ozcelikay G, Ozkan S. Carbon Dots in the Detection of Pathogenic Bacteria and Viruses. Critical Reviews in Analytical Chemistry. 2022;54(1):1–29. doi:10.1080/10408347.2022.2072168
234. Lee AC, Ye J, Tan S, et al. Carbon nanotube-based labels for highly sensitive colorimetric and aggregation-based visual detection of nucleic acids. Nanotechnology. 2007;18(18):455102. doi:10.1088/0957-4484/18/45/455102
235. Tang C, He Z, Liu H, et al. Application of magnetic nanoparticles in nucleic acid detection. J Nanobiotechnology. 2020;18(1):62. doi:10.1186/s12951-020-00613-6
236. Mabhude Y. Development of Gold Nanoparticles Based Lateral Flow Assay for Detection of Food and Water-Borne Pathogens. Universty of the Western Cape; 2024.
237. Huang R, Hu Q, Ko C-N, et al. Nano-based theranostic approaches for infection control: current status and perspectives. Materials Chemistry Frontiers. 2024;8(1):9–40. doi:10.1039/D3QM01048A
238. Jiang L, Ding L, Liu G. Nanoparticle formulations for therapeutic delivery, pathogen imaging and theranostic applications in bacterial infections. Theranostics. 2023;13(5):1545. doi:10.7150/thno.82790
239. Reygaert WC. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018;4(3):482–501. doi:10.3934/microbiol.2018.3.482
240. Prevention CfDCa. Antimicrobial Resistance. Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/antimicrobial-resistance/about/index.html.
241. Munita JM, Arias CA. Mechanisms of Antibiotic Resistance. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.VMBF-0016-2015
242. Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74(3):417–433. doi:10.1128/mmbr.00016-10
243. Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13(1):42–51. doi:10.1038/nrmicro3380
244. Hajipour MJ, Fromm KM, Ashkarran AA, et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10):499–511. doi:10.1016/j.tibtech.2012.06.004
245. Patel P, Hanini A, Shah A, et al. Surface Modification of Nanoparticles for Targeted Drug Delivery. BMC Complementary and Alternative Medicine. 2019;19(1):19–31. doi:10.1186/s12906-019-2428-5
246. Wu Z, Chan B, Low J, Chu JJH, Hey HWD, Tay A. Microbial resistance to nanotechnologies: an important but understudied consideration using antimicrobial nanotechnologies in orthopaedic implants. Bioact Mater. 2022;16:249–270. doi:10.1016/j.bioactmat.2022.02.014
247. Pelgrift RY, Friedman AJ. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev. 2013;65(13–14):1803–1815. doi:10.1016/j.addr.2013.07.011
248. Hemeg HA. Nanomaterials for alternative antibacterial therapy. Int J Nanomedicine. 2017;12:8211–8225. doi:10.2147/ijn.S132163
249. Morones-Ramirez JR, Winkler JA, Spina CS, Collins JJ. Silver enhances antibiotic activity against gram-negative bacteria. Sci Transl Med. 2013;5(190):190ra81. doi:10.1126/scitranslmed.3006276
250. Zhu X, Tang Q, Zhou X, Momeni MR. Antibiotic resistance and nanotechnology: a narrative review. Microb Pathog. 2024;193:106741. doi:10.1016/j.micpath.2024.106741
251. Garipov I, Khaydarov R, Gapurova O, et al. Silver Nanoparticles as a New Generation of Antimicrobial Prophylaxis. Journal of Siberian Federal University Biology. 2019:266–276. doi:10.17516/1997-1389-0301
252. AlQurashi DM, AlQurashi TF, Alam RI, Shaikh S, Tarkistani MAM. Advanced Nanoparticles in Combating Antibiotic Resistance: current Innovations and Future Directions. Journal of Nanotheranostics. 2025;6(2):9. doi:10.3390/jnt6020009
253. Li X, Robinson SM, Gupta A, et al. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano. 2014;8(10):10682–10686. doi:10.1021/nn5042625
254. Berdimurodov E, Hussain CM, Tüzün B. Multifunctional Magnetic Nanoparticles in Therapy, Biology, and Pharmacy: Synthesis, Fundamentals and Applications. CRC Press; 2024.
255. Mateo EM, Jiménez M. Silver nanoparticle-based therapy: can it be useful to combat multi-drug resistant bacteria? Antibiotics. 2022;11(9):1205. doi:10.3390/antibiotics11091205
256. Khan O, Chaudary N. The use of amikacin liposome inhalation suspension (Arikayce) in the treatment of refractory nontuberculous mycobacterial lung disease in adults. Drug Design, Development and Therapy. 2020;Volume 14:2287–2294. doi:10.2147/DDDT.S146111
257. Zeb A, Gul M, Nguyen -T-T-L, Maeng H-J. Controlled release and targeted drug delivery with poly (lactic-co-glycolic acid) nanoparticles: reviewing two decades of research. Journal of Pharmaceutical Investigation. 2022;52(6):683–724. doi:10.1007/s40005-022-00584-w
258. Rodrigues GR, López-Abarrategui C, de la Serna Gómez I, Dias SC, Otero-González AJ, Franco OL. Antimicrobial magnetic nanoparticles based-therapies for controlling infectious diseases. International Journal of Pharmaceutics. 2019;555:356–367. doi:10.1016/j.ijpharm.2018.11.043
259. Xie X, Yue T, Gu W, et al. Recent advances in mesoporous silica nanoparticles delivering siRNA for cancer treatment. Pharmaceutics. 2023;15(10):2483. doi:10.3390/pharmaceutics15102483
260. Zheng C, Li M, Ding J. Challenges and opportunities of nanomedicines in clinical translation. Bio Integration. 2021;2(2):57. doi:10.15212/bioi-2021-0016
261. Đorđević S, Gonzalez MM, Conejos-Sánchez I, et al. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Delivery Transl Res. 2022;20221–26.
262. Thapa RK, Kim JO. Nanomedicine-based commercial formulations: current developments and future prospects. Journal of Pharmaceutical Investigation. 2023;53(1):19–33. doi:10.1007/s40005-022-00607-6
263. Kunjachan S, Ehling J, Storm G, Kiessling F, Lammers T. Noninvasive Imaging of Nanomedicines and Nanotheranostics: principles, Progress, and Prospects. Chem Rev. 2015;115(19):10907–10937. doi:10.1021/cr500314d
264. Isibor PO. Regulations and policy considerations for nanoparticle safety. In: Environmental Nanotoxicology: Combatting the minute Contaminants. Springer; 2024:295–316.
265. Alshememry AK, Alsaleh NB, Alkhudair N, Alzhrani R, Alshamsan A. Recent nanotechnology advancements to treat multidrug-resistance pancreatic cancer: pre-clinical and clinical overview. Frontiers in Pharmacology. 2022;13:933457. doi:10.3389/fphar.2022.933457
266. Xu Q, Hu X, Wang Y. Alternatives to conventional antibiotic therapy: potential therapeutic strategies of combating antimicrobial-resistance and biofilm-related infections. Molecular Biotechnology. 2021;63(12):1103–1124. doi:10.1007/s12033-021-00371-2
267. Desai N, Rana D, Patel M, et al. Nanoparticle Therapeutics in Clinical Perspective: Classification, Marketed Products, and Regulatory Lands. Small. 2025;21:2502315. doi:10.1002/smll.202502315
268. Khatoon UT, Velidandi A. An Overview on the Role of Government Initiatives in Nanotechnology Innovation for Sustainable Economic Development and Research Progress. Sustainability. 2025;17(3):1250. doi:10.3390/su17031250