- Vivienne Westwood x Art of Heritage Spring 2026 at Riyadh Fashion Week WWD
- Vivienne Westwood Show Opens Riyadh Fashion Week, as Saudis Highlight Creative Side The New York Times
- Saudi labels ‘honor roots’ at Riyadh Fashion Week Arab News
Blog
-

Vivienne Westwood x Art of Heritage Spring 2026 at Riyadh Fashion Week – WWD
-
ESMO 2025: Overall Survival with Enzalutamide in Biochemically Recurrent Prostate Cancer – UroToday
- ESMO 2025: Overall Survival with Enzalutamide in Biochemically Recurrent Prostate Cancer UroToday
- Drug combo cuts risk of death in advanced prostate cancer by 40%, clinical trial finds Medical Xpress
- Pfizer Co & Astellas announce final overall…
Continue Reading
-

Amped Bikes brings kid-friendly e-mobility to EICMA 2025 – thepack.news
At EICMA 2025, British brand Amped Bikes will showcase its range of lightweight offroad electric bikes designed specifically for young riders. The lineup includes the A10, A16, and A20, aimed at children aged 3 to 12 years.
Continue Reading
Brensocatib Reduced Symptom Burden, Neutrophil Serine Proteases Activity in Bronchiectasis
Abstracts presented during the
CHEST annual meeting , held in Chicago, Illinois, October 19-22, 2025, showed the efficacy of brensocatib (Brinsupr; Insmed Incorporated Investor Relations) in reducing the activity of neutrophil serine proteases (NSPs) and reducing symptom burden in patients with non–cystic fibrosis bronchiectasis.Brensocatib was approved by the FDA for use in bronchiectasis just this year based on the findings of the ASPEN trial (
NCT04594369 ).1 Although the approval was solely in adults, other studies found that brensocatib was effective in adolescents as well, presenting the first-ever method of treatment for those living with bronchiectasis.An abstract reporting on the effect of brensocatib on sputum NSP concentrations compared with placebo through 52 weeks of treatment and 4 weeks of follow-up was presented during the conference.2 All participants were adult patients from the ASPEN trial and were required to have at least 2 instances of pulmonary exacerbations in the 12 months prior to screening for the trial. Participants were split 1:1:1 for 10-mg brensocatib, 25-mg brensocatib, or a matching placebo. Sputum samples were collected at baseline and weeks 4, 16, 28, 40, 52, and 56, with the last sample being collected 4 weeks after treatment.
There were 105 participants in the 10-mg group, 108 in the 25-mg group, and 115 in the placebo group who were included in the analysis. Neutrophil elastase (NE), cathepsin G (CatG), and proteinase 3 (PR3) were the primary NSPs evaluated and varied between the 3 groups at baseline.
Both groups receiving brensocatib saw a decrease in activity of NE, CatG, and PR3 by the fourth week compared with the placebo group, which did not see any change. The median (range) percent change in the 10-mg and 25-mg groups was –33.7% (–58.8% to 0.0%) and –41.2% (–71.1% to –3.6%), respectively, compared with –4.6% in the placebo group for NE; similar results were found for median change in CatG (–39.7%, –48.1%, and 0.0%, respectively) and PR3 (–27.7%, –58.4%, and 0.0%, respectively). When participants stopped taking treatment, activity returned to baseline.
The authors concluded that active receipt of brensocatib resulted in the reduction of activity in NSP within the first 4 weeks of taking the treatment. The 25-mg dose also resulted in a higher reduction in activity compared with the 10-mg dose.
The second abstract focused on the effect of brensocatib on symptom burden for patients with bronchiectasis with or without pulmonary exacerbations.3 The ASPEN trial had previously found that both the 10-mg and 25-mg doses of brensocatib had reduced the rate of pulmonary exacerbations, the time to first exacerbation, and increased the odds of going without an exacerbation for 52 weeks. This analysis instead focused on how brensocatib affected symptoms of those with bronchiectasis with or without pulmonary exacerbations using the Bronchiectasis Exacerbation and Symptom Tool (BEST).
Participants were all from the ASPEN trial randomized 1:1:1 to 10 mg of brensocatib, 25 mg of brensocatib, or placebo for 52 weeks. Severe exacerbations were defined as those that required intravenous antibiotics or hospitalization. The BEST measurement assessed breathlessness, fatigue, sputum volume, cough, symptoms of the cold or flu, and sputum purulence. These measurements were taken each day until the end of the study. Lower scores indicated fewer symptoms.
There were 784 participants in the group without exacerbations, 879 in the group with any exacerbation, and 194 in the group with severe exacerbations included in the study. Symptom burden was greater in patients with exacerbations compared with those without any exacerbations. Those who used brensocatib had a baseline decrease in BEST score that was greater than those who did not take brensocatib across all subgroups.
Those who took brensocatib had reduced symptom burden 21 days before and after a severe exacerbation compared with placebo, with a mean (SD) peak score increase from baseline of 3.45 (4.1) in the 10-mg group and 3.43 (4.4) in the 25-mg group that was taken 5 to 7 days before the start date of the severe exacerbation. In comparison, the mean peak score increase was 4.07 (4.4) in those who used placebo.
The researchers concluded that symptom burden was reduced in those taking brensocatib of either dose regardless of the occurrence of exacerbations. Those without exacerbations and patients using the 25-mg dose saw the greatest reductions in symptom burden.
References
1. McCormick B. Brensocatib becomes first FDA-approved therapy for bronchiectasis. AJMC®. August 12, 2025. Accessed October 15, 2025.
https://www.ajmc.com/view/brensocatib-becomes-first-fda-approved-therapy-for-bronchiectasis 2. Metersky ML, De-Soyza A, Burgel PR, et al. Effects of brensocatib on neutrophil serine protease levels in patients with noncystic fibrosis bronchiectasis: an analysis of the ASPEN trial. Presented at: CHEST; October 19-22, 2025; Chicago, Illinois.
3. Flume PA, Metersky ML, Mauger D, et al. The effect of brensocatib vs placebo on symptom burden in patients with or without on-study pulmonary exacerbations: a posthoc analysis from the ASPEN trial. Presented at: CHEST; October 19-22, 2025; Chicago, Illinois.
Continue Reading
WHO says DRC could declare end of Ebola outbreak by December-Xinhua
KINSHASA, Oct. 19 (Xinhua) — The World Health Organization (WHO) said Sunday that health authorities could declare the end of the current Ebola outbreak in the Democratic Republic of the Congo (DRC) in early December if no new cases are…
Continue Reading

KP governor, interior minister discuss terrorism, law and order

LAHORE, OCT 19 (APP/DNA): Governor Khyber Pakhtunkhwa Faisal Karim Kundi met with Federal Interior Minister Mohsin Naqvi at his residence in Lahore on Sunday to discuss the overall law and order situation in…
Continue Reading

The Imminent Solar Conjunction of 3I/ATLAS | by Avi Loeb | Oct, 2025
Press enter or click to view image in full size(Credit: NASA/JPL) On October 21, 2025, the interstellar object 3I/ATLAS will be exactly on the opposite side of the Sun relative to Earth, constituting a so-called `solar-conjunction’. When you…
Continue Reading
OPTIPRIME Trial at ESMO 2025: “Stop-and-Go” FOLFOX Plus Panitumumab Strategy Extends Disease Control in RAS/BRAF w/t mCRC
At the ESMO Congress 2025 in Berlin, Dr. Jean-Baptiste Bachet (Paris, France) presented the final results of the phase II OPTIPRIME trial (Abstract 727MO), a multicentre French study evaluating a novel “stop-and-go” strategy combining FOLFOX and panitumumab (Pmab) in patients with RAS/BRAF wild-type metastatic colorectal cancer (mCRC).
At OncoDaily GI, we spotlight the innovations reshaping colorectal cancer care — from adaptive treatment sequencing to intelligent use of targeted therapies to mitigate resistance and cumulative toxicity.
Background
Epidermal growth factor receptor (EGFR) blockade has long been a cornerstone of therapy in RAS/BRAF wild-type mCRC, delivering significant response rates and survival benefits. Yet, the cumulative toxicities associated with prolonged anti-EGFR exposure — particularly dermatologic and nail toxicities — often limit treatment duration. Furthermore, continuous EGFR inhibition can foster resistant subclones, potentially diminishing long-term efficacy.
The OPTIPRIME trial was designed to test whether an intermittent (“stop-and-go”) use of panitumumab, alongside oxaliplatin-based chemotherapy, could maintain efficacy while improving tolerability and extending the duration of disease control (DDC) — a metric capturing both the initial and subsequent treatment phases under EGFR-targeted therapy.
Study Design and Methods
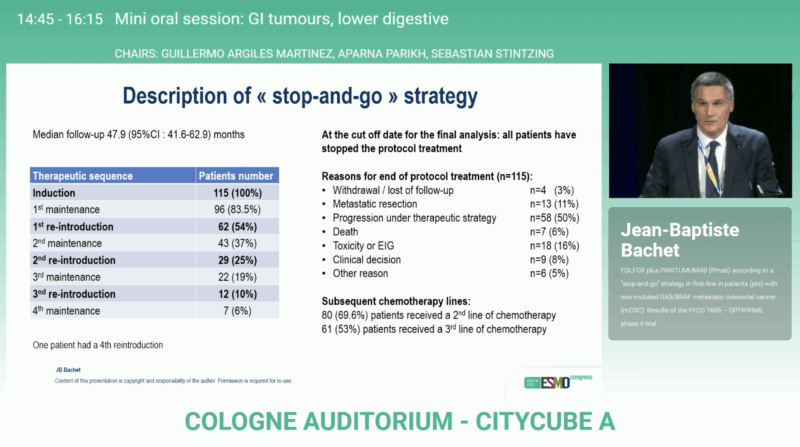
Conducted across 36 French centers between April 2018 and May 2023, OPTIPRIME enrolled 118 patients (mITT: 115 after excluding 3 with RAS or BRAF mutations). Eligible patients had previously untreated, measurable, RAS/BRAF wild-type mCRC with ECOG performance status 0–1.
Treatment consisted of
- Induction phase: 6 cycles of FOLFOX plus panitumumab
- Maintenance phase: Fluoropyrimidine monotherapy (LV5FU2 or capecitabine) for patients achieving disease control
- Reintroduction phase: Upon progression, reintroduction of panitumumab ± oxaliplatin for 6 cycles, repeated as needed (“stop-and-go”)
The primary endpoint, DDC, was defined as the time from inclusion to radiological progression under EGFR therapy with chemotherapy or death. Patients undergoing R0/R1 metastasectomy were censored at surgery, and those switching therapy without progression were censored at the time of switch.
The study was powered to detect an improvement from a median DDC of 14 months (H0) to 20 months (H1), with a 1-sided α of 5% and 80% power. The predefined threshold for success (upper critical value) was 17.84 months.
Results
After a median follow-up of 47.9 months, OPTIPRIME achieved its primary endpoint with a median DDC of 24.9 months (90 % CI 19.3–28.3), surpassing the predefined efficacy boundary. The overall response rate reached 74.8 %, reflecting substantial activity of the FOLFOX + panitumumab regimen.
Most patients (83.5 %) entered at least one maintenance phase, and many underwent repeated reintroductions of panitumumab, illustrating the practical feasibility of this cyclic regimen. A subset (11 %) proceeded to metastasectomy with curative intent. At data cut-off, 69.6 % of patients had received subsequent second-line therapy.
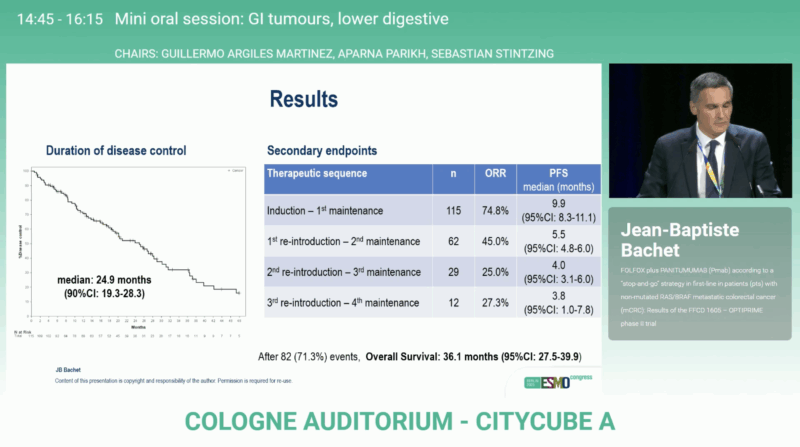
The median overall survival was 36.1 months (95 % CI 27.5–39.9), among the best reported for first-line mCRC in this molecular subgroup. Median progression-free survival (PFS) during the induction and first maintenance phase was 9.9 months (95% CI 8.3–11.1), confirming durable early disease control. These findings confirm that intermittent EGFR inhibition can sustain long-term disease control while preserving patient quality of life and delaying resistance.
Interpretation
The OPTIPRIME trial successfully met its primary endpoint, validating a biologically rational and clinically practical strategy for optimizing EGFR-targeted therapy in mCRC. The median DDC of nearly 25 months underscores the durability achievable through treatment holidays, allowing patients to recover from toxicity while preserving future sensitivity to anti-EGFR agents.
This “stop-and-go” model represents an important step forward in adaptive treatment design, addressing one of the key dilemmas in metastatic colorectal cancer: how to balance intensity, tolerance, and long-term benefit. It aligns with growing evidence from molecular studies showing that resistant EGFR clones can decay during drug-free intervals, restoring sensitivity upon rechallenge.
Clinical Implications
The OPTIPRIME findings strengthen the case for flexible, individualized sequencing of anti-EGFR therapy in mCRC. By alternating active and maintenance phases, clinicians can maintain high disease control rates while minimizing cumulative toxicity and optimizing quality of life.
With median survival exceeding three years, the study underscores how thoughtful integration of targeted agents within an adaptive framework can meaningfully extend outcomes in metastatic colorectal cancer.
You can read the full abstract here.
Conclusion
The OPTIPRIME phase II trial (NCT03584711) validates the clinical value of a “stop-and-go” panitumumab strategy in RAS/BRAF wild-type mCRC. By integrating periods of EGFR inhibition and maintenance fluoropyrimidine therapy, investigators achieved both prolonged disease control and strong overall survival, with improved tolerability.
These results may inform future treatment paradigms emphasizing adaptive, toxicity-conscious sequencing of targeted agents — a principle likely to gain further traction as precision oncology continues to evolve.

You can read about PERISCOPE II Trial: Randomized study finds no OS advantage for gastrectomy plus CRS/HIPEC versus systemic therapy on OncoDaily.
Continue Reading
Ruud rules in Stockholm, defeats Humbert in final – ATP Tour
- Ruud rules in Stockholm, defeats Humbert in final ATP Tour
- Tennis, ATP – Stockholm Open 2025: Ruud takes out Shapovalov tennismajors.com
- Ugo Humbert vs Casper Ruud Prediction: Can Humbert’s Indoor Fire Dethrone Ruud’s Baseline Mastery? Telecom…
Continue Reading

KP Governor, Mohsin Naqvi review security, anti-terror efforts
Governor Khyber Pakhtunkhwa Faisal Karim Kundi met with Federal Interior Minister Mohsin Naqvi at his residence in Lahore on Sunday to discuss the overall law and order situation in the province and recent incidents of terrorism.
During the…
Continue Reading