This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Blog
-
Just a moment…
Just a moment… -
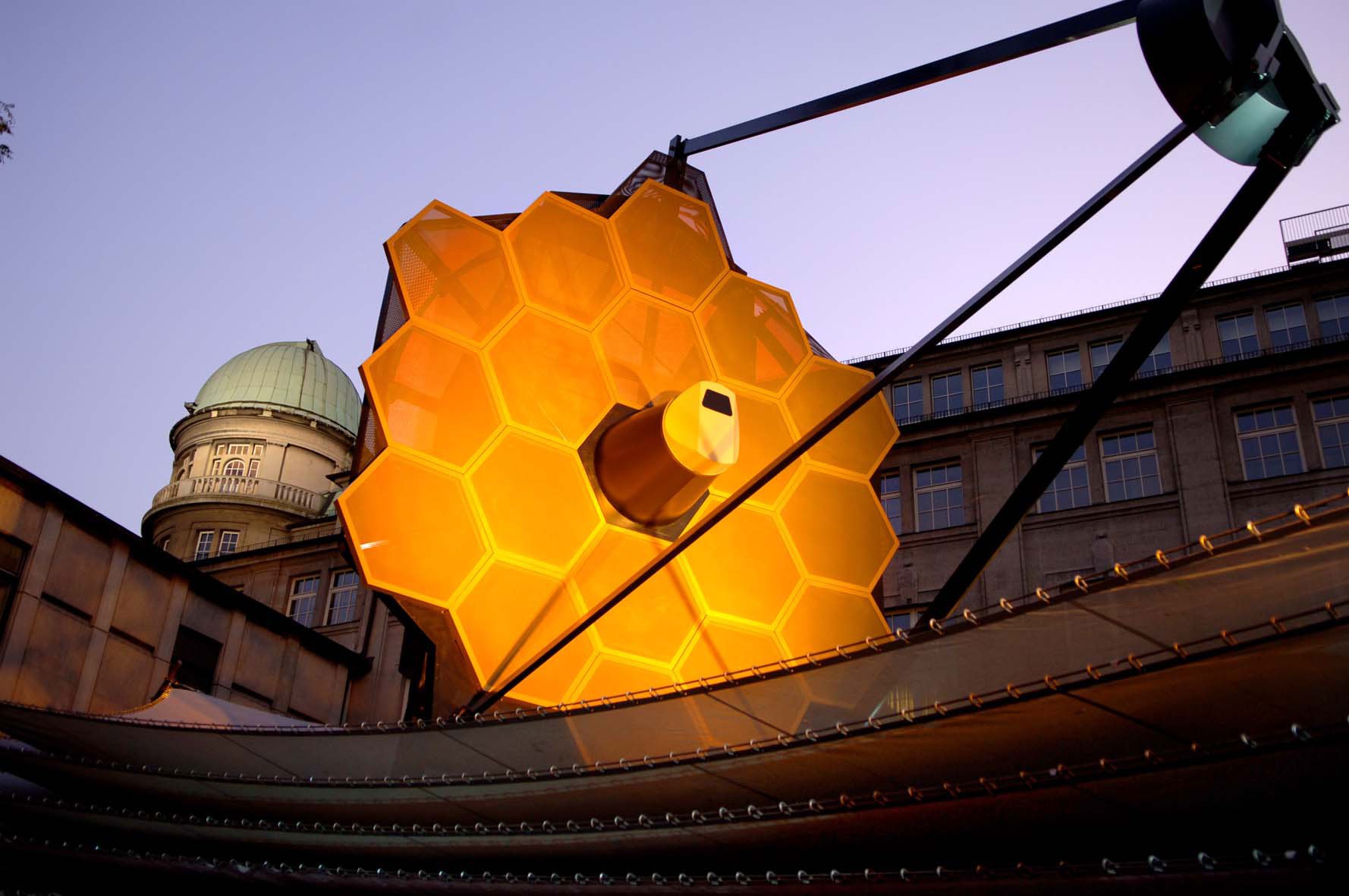
How A Trick From Radio Astronomy Could Help Astronomers Find Earth-like Planets
The wavelengths of radio light are so large that you can’t capture a high-resolution image with a single dish. To capture an image as sharp as, say, the Hubble telescope, you’d need a radio dish tens of kilometers across. So radio…
Continue Reading
-
Just a moment…
Just a moment… This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Continue Reading
-

Disney+ and Hulu cancellation rates doubled after Kimmel suspension | Jimmy Kimmel
Disney’s short-lived suspension of Jimmy Kimmel under pressure from the Trump administration may have had a permanent impact on the company’s subscription numbers.
According to data released by Antenna, an analytics firm that tracks…
Continue Reading
-

The British Museum hosts its first-ever ball, attended by Mick Jagger and Naomi Campbell.
The British Museum held its inaugural ball on October 18th, marking the launch of a new annual event spotlighting London’s status as a global cultural capital. Co-chaired by arts patron and businesswoman Isha Ambani and the museum’s director,…
Continue Reading
-
ESMO 2025: IMvigor011: A Phase 3 Trial of ctDNA-Guided Adjuvant Atezolizumab versus Placebo in MIBC – UroToday
- ESMO 2025: IMvigor011: A Phase 3 Trial of ctDNA-Guided Adjuvant Atezolizumab versus Placebo in MIBC UroToday
- Chugai Pharmaceutical : Roche’s Announcement Regarding Tecentriq (Presentation of Latest Data as an Adjuvant Treatment in Muscle-Invasive Bladder Cancer at ESMO) MarketScreener
- IMvigor011: ctDNA-Guided Adjuvant Atezo in Bladder Cancer Oncodaily
- Genentech’s Tecentriq Showed Significant Overall and Disease-Free Survival Benefits in Bladder Cancer With ctDNA-Guided Treatment Yahoo Finance
- Blood Test Directs Post-Surgery Immunotherapy for Bladder Cancer Mirage News
Continue Reading
-
Just a moment…
Just a moment… This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Continue Reading
-

How to watch NBA games in 2025-26: Everything you need to know
With the 2024-25 NBA schedule officially released in full, check out this breakdown by the numbers.
• Download the NBA App
• Amex 2025-26 Season PreviewThe 2025-26 NBA season brings new broadcast partners and new ways to watch games all…
Continue Reading
-

FDA Issues CRL for Dasatinib in CML/ALL
The FDA has given a complete response letter (CRL) to dasatinib (Dasynoc) for patients with chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL), according to a press release from Xspray Pharma, the drug’s developer.1
Based on Good Manufacturing Practice observations at the manufacturer for the drug, the FDA elected to provide a CRL. Although no direct observations were made for the production of dasatinib, the FDA is pausing all approvals at the facility until corrective actions are taken. Currently, an action plan on steps to remediate these observations is underway. An additional meeting is scheduled with the FDA for December.
“It is unfortunate that manufacturing-related issues beyond our control are delaying our launch. We have made significant progress in the regulatory review and maintained discussions with the FDA regarding the product information for [dasatinib] up to the [Prescription Drug User Fee Act] date,” Per Andersson, chief executive officer of of Xspray Pharma, said in the press release.1 “We will now work closely with both the manufacturer and the FDA to expedite the process and enable a resubmission as soon as the corrective actions have been completed.”
Dasatinib was designed using Xspray’s patented HyNap technology, which has been leveraged for proven safety and efficacy outcomes.2 This amorphous formulation of dasatinib is dosed 30% lower. The bioequivalence allows for patients to be given doses more accurately to get a higher benefit from treatment. Amorphous dasatinib can be prescribed with any acid-reducing agent like proton pump inhibitors, H2 antagonists, or antacids.
What Was the Path to Approval?
In July 2024, dasatinib received a CRL from the FDA.2 This CRL was based on an updated new drug application (NDA) submitted in February 2024.3 The CRL outlined a need for additional information regarding the labeling comprehension and pre-approval inspection at the third-party manufacturing site. Inspection occurred from June 10 to 19, 2024. However, the FDA did not request additional clinical studies and did not question any submitted clinical data.
In April 2025, an updated NDA was submitted addressing all the FDA’s previous concerns.4
“Manufacturing and quality review of new tablet batches, which were required to address the FDA’s questions, have gone according to plan at Xspray’s US contract manufacturer, and live up to all the set quality requirements,” Andersson said in the press release regarding the resubmitted NDA.4 “We are well prepared to launch [dasatinib] on the US market upon approval later this year,” he concluded.
According to the prescribing information for dasatinib tablets (Sprycel), the agent is currently indicated for the treatment of patients with newly diagnosed Philadelphia chromosome (Ph)–positive CML in chronic phase; chronic, accelerated, or myeloid or lymphoid blast phase Ph-positive CML with resistance or intolerance to prior therapy, including imatinib (Gleevec); and Ph-positive ALL with resistance or intolerance to prior treatment.5 The agent is also indicated for use in pediatric patients who are 1 year and older with Ph-positive CML in chronic phase and newly diagnosed Ph-positive ALL in combination with chemotherapy.
The prescribing information for dasatinib tablets includes warnings for myelosuppression and bleeding events, fluid retention, cardiovascular toxicity, pulmonary arterial hypertension, severe dermatologic reactions, and tumor lysis syndrome. Additionally, the most common adverse effects associated with the agent in pediatric patients include mucositis, febrile neutropenia, pyrexia, diarrhea, nausea, vomiting, abdominal pain, cough, headache, rash, fatigue, constipation, edema, hypertension, and infections.
References
- Xspray Pharma provides update on the FDA process for Dasynoc-observations at contract manufacturer delay approval. News release. Xspray Pharma. October 8, 2025. Accessed October 20, 2025. https://tinyurl.com/yc366ske
- Xspray Pharma shares new information on Dasynoc, a novel CML treatment in development. News release. Xspray Pharma. July 24, 2024. Accessed September 30, 2025. https://tinyurl.com/mvmr48de
- FDA Accepts Xspray Pharma’s NDA-resubmission for Dasynoc® – PDUFA Date set to 31 July 2024. News release. Xspray Pharma. February 12, 2024. Accessed September 30, 2025. https://tinyurl.com/9spn57dh
- Xspray Pharma re-submits its FDA application. News release. Xspray Pharma. April 8, 2025. Accessed September 30, 2025. https://tinyurl.com/42v75swu
- SPRYCEL® (dasatinib) tablets, for oral use. Prescribing information. FDA; Revised July 2024. Accessed October 20, 2025. https://tinyurl.com/mwcbnmf9
Continue Reading
-

The Athletic: For Anthony Edwards, an NBA title is just one step away. Inside his ‘unstoppable’ plan
From Watkins’ viewpoint, that was part of the problem. Football was too easy for Edwards. When he joined a higher level team, serendipitously called the Vikings, he hated the extra conditioning and practicing that the coaches demanded of…
Continue Reading