TikToker Steve Bridges has passed away at age 41.
His wife, Chelsey Bridges, shared the news on his Instagram page on Friday, Oct. 17.
“A light gone too soon. I love you Steve,” she captioned her video, in which she revealed that the Illinois…

TikToker Steve Bridges has passed away at age 41.
His wife, Chelsey Bridges, shared the news on his Instagram page on Friday, Oct. 17.
“A light gone too soon. I love you Steve,” she captioned her video, in which she revealed that the Illinois…
The addition of BNT111 to cemiplimab-rwlc (Libtayo) led to an objective response rate (ORR) of 18.1% (95% CI, 10.9%-27.4%; P = .0115) in patients with PD-(L)1-relapsed/refractory melanoma, allowing investigators to reject the null hypothesis of an ORR below 10%, according to data from the phase 2 BNT111-01 trial (NCT04526899) that were presented at the
Best responses included a complete response (CR) rate of 11.7%, partial response (PR) rate of 6.4%, and stable disease (SD) rate of 37.2%. The disease control rate (DCR) was 55.3% (95% CI, 44.7%-65.6%).
With median follow-up of 15.7 months (range, 0.2-42.2), the median progression-free survival (PFS) was 3.1 months (95% CI, 1.7-6.9); the 24-month PFS rate was 24.9% (95% CI, 14.9%-36.1%). The median overall survival (OS) was 20.7 months (95% CI, 14.4-28.3); the 24-month OS rate was 47.8% (95% CI, 36.4%-58.4%).
“The results indicated statistically significant improvement of BNT111 plus cemiplimab vs an assumed historical control ORR of 10% in heavily pretreated, PD-(L)1-relapsed/refractory advanced or metastatic cutaneous non-acral melanoma,” lead study author Paolo Ascierto, MD, full professor of oncology at the University of Napoli Federico II, and director of the Department of Melanoma, Cancer Immunotherapy and Development Therapeutics at the Istituto Nazionale Tumori IRCCS Fondazione Pascale in Naples, Italy, said in a presentation.
The company announced
BNT111 is an investigational uridine RNA-based lipoplex cancer immunotherapy targeting the nonmutated, tumor-associated antigens NY-ESO-1, MAGE-A3, Tyrosinase, and TPTE.1
BNT111-01 was designed as an open-label, randomized, multi-center, interventional trial to evaluate the activity and safety of BNT111 plus cemiplimab as second-line therapy in patients with unresectable stage III or IV melanoma who had progressed on prior PD-(L)1 therapy.
To be eligible for the trial patients had to have measurable disease, serum lactase dehydrogenase levels below the upper limit of normal, and received up to 5 prior lines of therapy including ipilimumab (Yervoy). Notably, patients had to enter the trial within 6 months of confirmed disease progression and have received at least 12 weeks of treatment, which must have included a BRAF-based combination for patients with BRAF-mutant disease.
A total of 180 patients were randomly assigned 2:1:1 to treatment with BNT111 plus cemiplimab (n = 94; arm 1), BNT111 monotherapy (n = 46; arm 2), or cemiplimab monotherapy (n = 44; arm 3), all for up to 24 months. Patients in the monotherapy arms were allowed to add the other agent upon confirmation of disease progression.
The primary end point was ORR by blinded independent central review per RECIST 1.1 in arm 1, which was compared with an assumed historic control ORR of 10%. Secondary end points were ORR in arms 2 and 3, duration of response, DCR, time to response, PFS, OS, safety, tolerability, and patient-reported outcomes.
Patients were followed for safety for 90 days and OS every 3 months for up to 48 months from the last randomization.
Baseline characteristics of the combination arm revealed that the median age was 64.0 (range, 18-84) and most patients were male (63.8%) and had an ECOG performance status of 0 (78.7%). The majority also had stage IV disease at baseline (97.9%), M1c disease (44.7%), and between 2 and 5 prior therapies (56.4%). Patients also were PD-(L)1 refractory (56.4%), and had liver metastases (25.5%), BRAF V600 mutations (28.7%), prior BRAF/MEK therapy (18.1%), and prior ipilimumab (48.9%).
“All patients were PD-(L)1 relapsed/refractory and had received one or multiple prior therapies with half of patients being CTLA4 pretreated,” Ascierto said.
“BNT111 also indicated clinical activity as monotherapy.” The ORR was 17.4% (95% CI, 7.8%-31.4%) with BNT111 monotherapy (n = 46), which included best responses of CR (13.0%), PR (4.3%), and SD (41.3%). The DCR was 58.7% (95% CI, 43.2%-73.0%). With cemiplimab monotherapy (n = 44), the ORR was 13.6% (95% CI, 5.2%-27.4%), with best responses of CR (4.5%), PR (9.1%), and SD (34.1%). The DCR was 47.7% (95% CI, 32.5%-63.3%).
Median follow-up was 11.8 months (range, 0.6-38.4) in the BNT111 arm and 16.9 months (range, 1.9-39.5) in the cemiplimab arm. In the BNT111 monotherapy arm, the median PFS was 2.8 months (95% CI, 2.6-4.7); the 24-month PFS rate was 20.9% (95% CI, 8.0%-37.9%). The median OS was 13.7 months (95% CI, 10.2-24.6); the 24-month OS rate was 37.6% (95% CI, 22.7%-52.5%). In the cemiplimab monotherapy arm, the median PFS was 3.2 months (95% CI, 1.5-5.0); the 24-month PFS rate was 10.6% (95% CI, 1.1%-32.5%). The median OS was 22.3 months (95% CI, 14.6-33.2); the 24-month OS rate was 43.1% (95% CI, 26.5%-58.7%).
With respect to safety in the combination arm (n = 92) treatment-emergent adverse effects (TEAEs) associated with cytokine release included pyrexia (any grade, 76.1%; grade ≥3, 0%), hypertension (any grade, 12.0%; grade ≥3, 6.5%), hypotension (any grade, 13.0%; grade ≥3, 2.2%), fatigue (any grade, 25.0%; grade ≥3, 3.3%), cytokine release syndrome (any grade, 8.7%; grade ≥3, 1.1%), and chills (any grade, 51.1%; grade ≥3, 0%).
TEAEs leading to dose reduction (9.8%), interruption (29.3%), and discontinuation (6.5%) also occurred. TEAEs occurred in 98.9% of cases and were deemed related to BNT111 in 97.8% (grade ≥3, 18.5%) and cemiplimab in 71.7% (grade ≥3, 14.1%).
“BNT111, both as a monotherapy and in combination therapy, exhibited a manageable safety profile, which is primarily driven by the induction of cytokines through toll-like receptors by the single-stranded RNA immunotherapy,” Ascierto said in conclusion.
Disclosures: Ascierto disclosed serving as a consultant or advisory role for Bristol-Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, Merck Serono, Pierre-Fabre, Sun Pharma, Immunocore, Italfarmaco, Boehringer-Ingelheim, Regenerson, Pfizer, Nouscom, Lunaphore, Medicenna, Bio-Al Health, ValoTx, Replimune, Bayer, Erasca, Philogen, BioNTech, Anaveon, Genmab, Menarini, Incyte, and ImCheck Therapeutics; research funding from Bristol-Myers Squibb, Roche-Genentech, Pfizer, Regeneron, and Medicenna; travel support from Pfizer, Bio-Al Health, Replimune, MSD, Pierre Fabre, and Philogen; and non-financial interests as president of the Fondazione Melanoma Onlus, president of the Campania Society of ImmunoTherapy of Cancer, member of the steering committee of the Society of Melanoma Research, and a member of the Board of Directors for the Society of Immuno-Therapy of Cancer.

Hello! I’m Liz Angell, an editor on the Pursuits team, here this week to persuade you to redesign your engagement ring.
Lots of jewelry has symbolic and sentimental value, but perhaps no piece has more than an engagement ring. But yours might…
Rain scuppered any hope of a result in Colombo as New Zealand’s clash with Pakistan on Saturday was abandoned – a result that guarantees South Africa’s place in the Women’s Cricket World Cup 2025 semi-finals.
With eight points to their…

In the most highly anticipated opening of the season, the Fondation Cartier for Contemporary Art will unveil its new Parisian address – and it’s not…

Prime Minister Shehbaz Sharif has lauded Pakistan’s progress toward economic stability and diplomatic success, attributing it to effective governance, hard work, and a shared national vision.
Speaking to the media in Jahanian, Punjab, the…

Six-time champions Argentina will take on Morocco in the FIFA U-20 World Cup 2025 final at Chile’s Estadio Nacional Julio Martínez Prádanos in the early hours of Monday morning.
The Argentina vs Morocco FIFA U-20 World Cup 2025 final will be…

DESTINY-Breast05 (NCT04622319), presented by Dr. Charles E. Geyer (Pittsburgh, United States of America) at the ESMO Congress 2025, is a pivotal phase 3, open-label, randomized trial evaluating trastuzumab deruxtecan (T-DXd) versus the standard-of-care trastuzumab emtansine (T-DM1) in patients with HER2-positive early breast cancer (eBC) who had residual invasive disease after neoadjuvant therapy. The study was designed to determine whether T-DXd could improve long-term outcomes for this high-risk population compared with T-DM1, the established post-neoadjuvant standard of care
Patients with HER2-positive early breast cancer who have residual invasive disease following neoadjuvant chemotherapy and anti-HER2 therapy face a high risk of recurrence, particularly distant relapse. T-DM1 became the standard post-neoadjuvant treatment following the KATHERINE trial, but outcomes remain suboptimal for patients with high residual disease burden. Trastuzumab deruxtecan (T-DXd), a next-generation HER2-directed antibody–drug conjugate with a potent topoisomerase I inhibitor payload, has shown marked efficacy in metastatic settings, prompting investigation into its use in the early disease setting to reduce recurrence risk.
In DESTINY-Breast05, 1,635 patients with HER2-positive eBC and residual invasive disease after neoadjuvant taxane-based chemotherapy and HER2-targeted therapy were randomized 1:1 to receive either:
Eligible patients were considered high risk for recurrence, defined by clinical stages T4, N0–3, M0 or T1–3, N2–3, M0 at presentation, or residual nodal disease after neoadjuvant therapy.
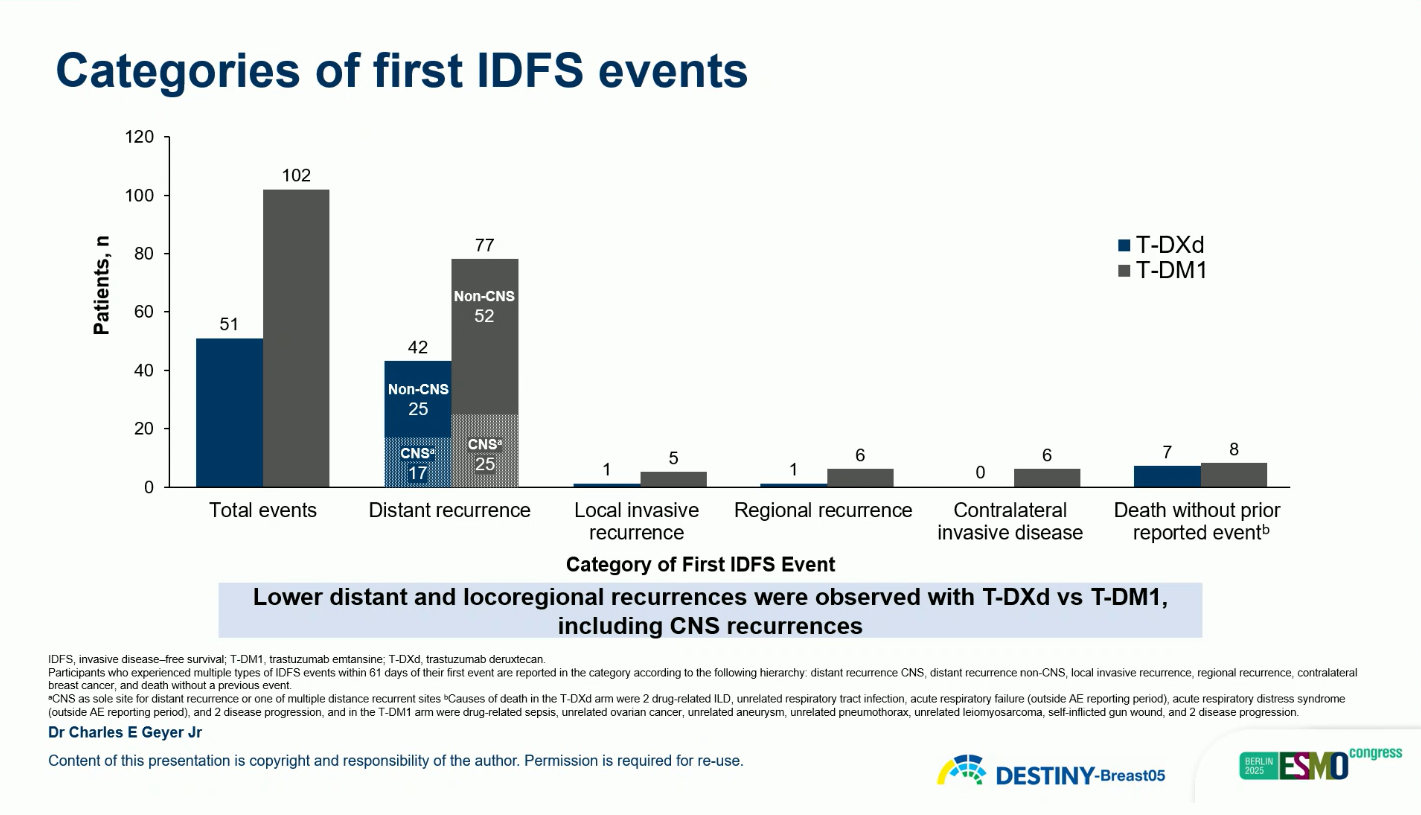
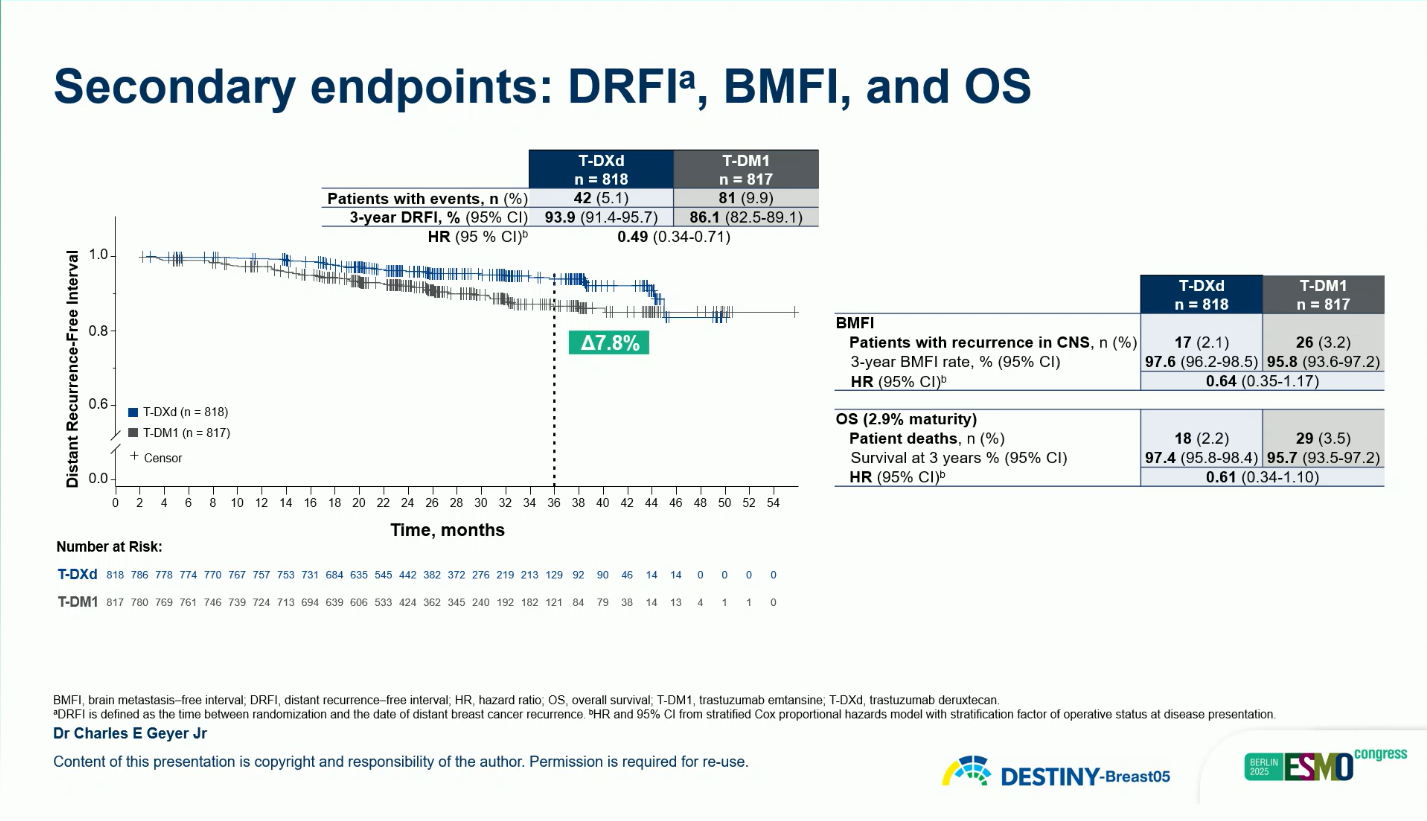
The primary endpoint was invasive disease-free survival (IDFS), with disease-free survival (DFS) as a key secondary endpoint. Additional endpoints included overall survival (OS), distant recurrence-free interval, brain metastasis–free interval (BMFI), and safety.
At the data cutoff of July 2, 2025, median follow-up was 29.9 months in the T-DXd arm and 29.7 months in the T-DM1 arm.
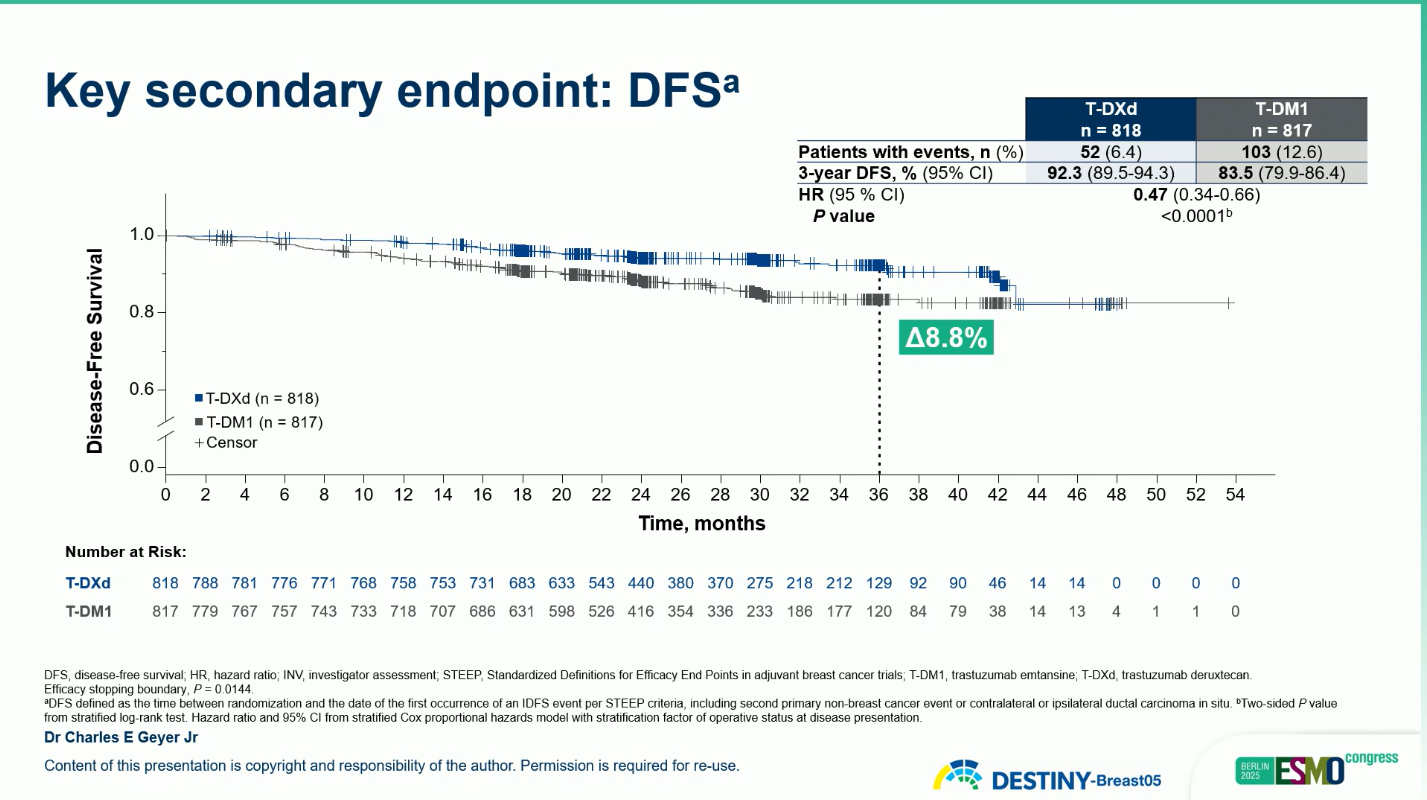
Similarly, the key secondary endpoint of disease-free survival (DFS) demonstrated a parallel benefit, with a hazard ratio of 0.47 (95% CI, 0.34–0.66; p < 0.0001). The 3-year DFS rate was 92.3% with T-DXd compared with 83.5% with T-DM1, corresponding to an 8.8% absolute gain.
These findings represent a 53% reduction in risk of invasive disease recurrence or death with T-DXd compared with T-DM1.
A clinically meaningful improvement in BMFI was also observed (HR 0.64; 95% CI 0.35–1.17), suggesting enhanced control of central nervous system relapse.
The overall safety profile of T-DXd was manageable and consistent with prior studies.
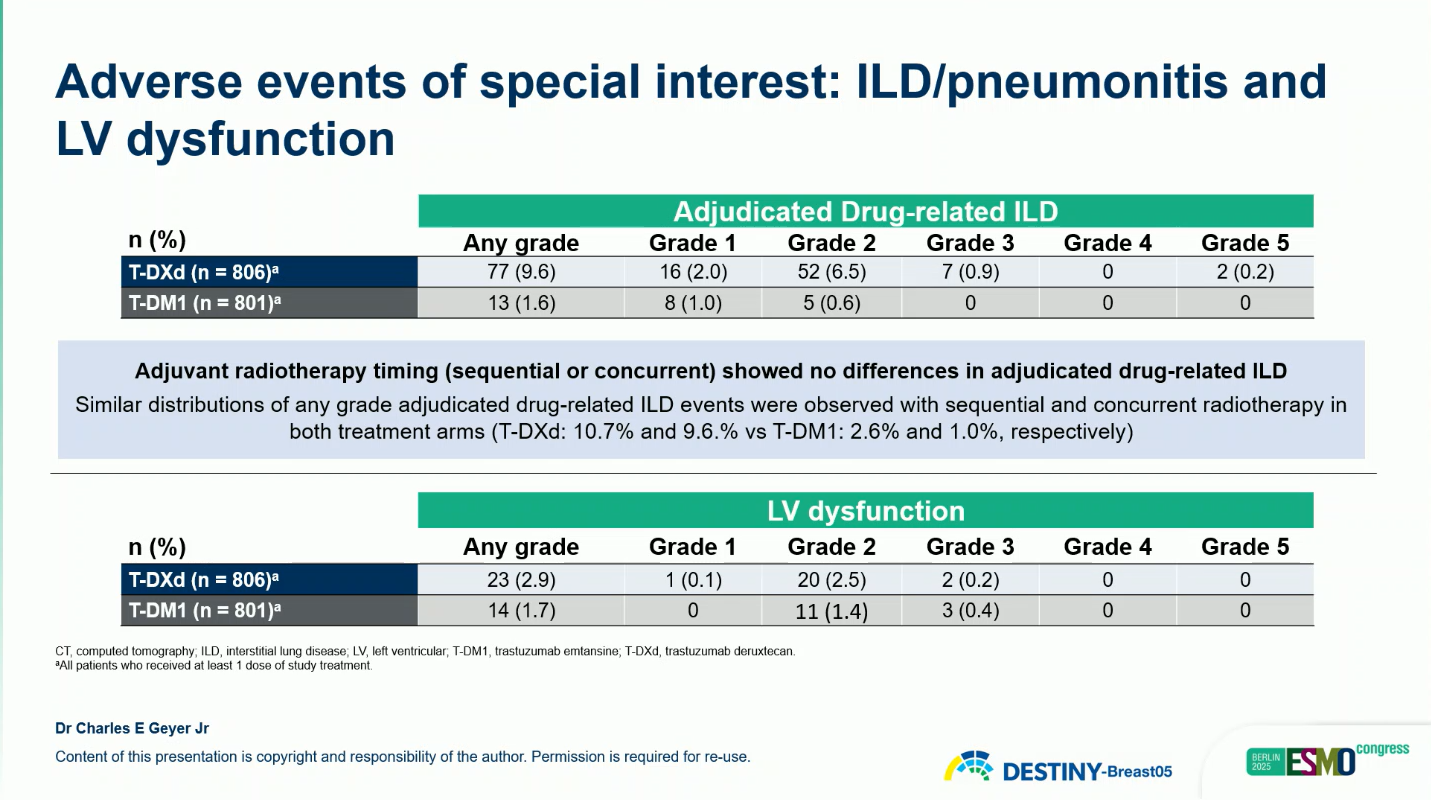
Most ILD events were grade 1–2 and resolved with treatment modification or corticosteroids. No new safety signals were identified.
The DESTINY-Breast05 trial demonstrated that trastuzumab deruxtecan (T-DXd) offers a statistically significant and clinically meaningful improvement in both invasive disease-free survival (IDFS) and disease-free survival (DFS) compared with trastuzumab emtansine (T-DM1) in patients with HER2-positive early breast cancer who had residual invasive disease following neoadjuvant therapy.
These findings mark a pivotal advance in the post-neoadjuvant management of HER2-positive breast cancer. By extending the proven efficacy of T-DXd beyond the metastatic setting into early-stage, high-risk disease, the results highlight its potential to redefine the standard of care for patients who previously had limited options after incomplete response to neoadjuvant therapy. Importantly, the benefit was consistent across all major subgroups, including hormone receptor–positive and –negative disease, as well as across regions and baseline disease characteristics, underscoring the robustness of the findings.
You can read the full abstract here.

Watch a room of 70-somethings when “Mrs. Robinson” starts. Shoulders straighten, eyes soften, lips move to words unvisited for decades. For three minutes and thirty seconds, mortgages and medications vanish. They’re nineteen again,…

San Diego, CA. October 17, 2025. Posing for the AI Camera at Twitch Con.
Charlie Fink
At TwitchCon San Diego, the Israeli startup Decart staged a live demonstration of something I’ve not seen before: real-time generative AI video. Partnering…