Previously, when Germans wanted to point out how crazy something is, they would naturally use German and say “Das ist verrückt.” Young Germans, however, now prefer to say, “Das crazy.”
Whether you’re surprised, speechless, want to make an ironic…

Previously, when Germans wanted to point out how crazy something is, they would naturally use German and say “Das ist verrückt.” Young Germans, however, now prefer to say, “Das crazy.”
Whether you’re surprised, speechless, want to make an ironic…



Booking travel is expensive these days, from the cost of trains or planes to paying for your luggage, lodgings and more. It’s important to have a backup plan in case things take a turn for the worst.
Booking your journey with a credit card that has travel insurance can ensure you’re covered in a number of different events. While many of these cards offer similar and overlapping benefits, some are better than others.
Below, we cover the best credit cards for travel protections and how they can provide you with peace of mind.
The Chase Sapphire Preferred® Card packs a punch for a $95 annual fee card, offering annual travel credits, comprehensive travel protections and more.
Highlights shown here are provided by the issuer and have not been reviewed by CNBC Select’s editorial staff.
Either $5 or 5% of the amount of each transfer, whichever is greater
Additional travel insurance perks: The Sapphire Preferred also comes with primary auto rental coverage, baggage delay insurance, lost luggage reimbursement, roadside assistance, travel accident insurance, travel and emergency assistance and trip delay insurance.
The Chase Sapphire Reserve® is a standout premium credit card with plenty of luxury perks and statement credits to justify its annual fee.
Highlights shown here are provided by the issuer and have not been reviewed by CNBC Select’s editorial staff.
Either $5 or 5% of the amount of each balance transfer, whichever is greater
Additional travel insurance perks: The Sapphire Reserve also comes with primary auto rental coverage, baggage delay insurance, emergency evacuation and transportation benefits, emergency medical and dental benefits, lost luggage reimbursement, roadside assistance, travel accident insurance, travel and emergency assistance services, and trip cancellation and interruption insurance.
The Capital One Venture X Rewards Credit Card is a premium credit card with a myriad of benefits and a lower annual fee than other high-end cards with similar features.
Highlights shown here are provided by the issuer and have not been reviewed by CNBC Select’s editorial staff.
$0 at the Transfer APR, 4% of the amount of each transferred balance that posts to your account at a promotional APR that Capital One may offer to you
Additional travel insurance perks: Alongside the Venture X card’s auto rental insurance, it offers lost luggage reimbursement, trip delay reimbursement, trip cancellation and interruption insurance, travel and emergency assistance and travel accident insurance.
The Citi Strata Elite℠ Card aims to brings premium restaurant rewards and travel benefits to users, including an annual hotel benefit of $300.
Highlights shown here are provided by the issuer and have not been reviewed by CNBC Select’s editorial staff.
Balance transfer fee applies with this offer 5% of each balance transfer; $5 minimum.
Additional travel insurance perks: Other travel insurance benefits of the Citi Strata Elite include secondary auto rental coverage (primary outside your country of residence), trip delay protection and trip cancellation and interruption protection.
On First Tech Federal Credit Union’s site
Earn 2X points on groceries, gas, electronics, medical, household goods and telecommunications, 1X points on all other purchases
Earn 20,000 points when you spend $3,000 in the first 60 days from account opening
Standout travel insurance benefit: The Choice Rewards World Mastercard isn’t a premium travel card, but it’s easier to get, has no annual fee and is still loaded with travel insurance perks. So if you can’t qualify for the top-tier travel credit cards or don’t want to shell out hundreds of dollars every year for a credit card with travel insurance, this is a great option.
Additional travel insurance perks: The Choice Rewards Mastercard offers baggage delay coverage, trip cancellation coverage, secondary MasterRental car coverage, travel accident insurance and lost or damaged luggage insurance.
| Credit card | Annual fee | CNBC Select’s pick for | Additional travel insurance benefits | Offers a welcome bonus |
|---|---|---|---|---|
| Chase Sapphire Preferred® Card | $95 | Best for trip cancellation and interruption insurance | Auto rental coverage, baggage delay insurance, lost luggage reimbursement, roadside dispatch, travel accident insurance, travel and emergency assistance services, trip delay reimbursement and trip cancellation and interruption insurance | Yes |
| Chase Sapphire Reserve® Credit Card | $795 | Best for trip delay reimbursement | Auto rental coverage, baggage delay insurance, emergency evacuation and transportation, lost baggage reimbursement, roadside assistance, trip delay reimbursement, travel accident insurance, travel and emergency assistance services, emergency medical and dental benefits and trip cancellation and interruption insurance | Yes |
| Capital One Venture X Rewards Credit Card | $395 | Best for auto rental coverage | Auto rental coverage, lost baggage insurance, travel accident insurance, trip delay coverage, travel and emergency assistance services and trip cancellation and interruption insurance | Yes |
| Citi Strata Elite℠ Card | $595 | Best for lost/damaged baggage | Auto rental coverage, lost/damaged baggage insurance, trip delay coverage and trip cancellation insurance | Yes |
| First Tech Credit Union Choice Rewards World Mastercard® | $0 | Low credit | MasterRental auto coverage, lost/damaged baggage insurance, baggage delay insurance, travel accident coverage and trip cancellation insurance | Yes |
The first step toward using your credit card travel insurance is making sure that you’re covered under its policy. Some cards offer all types of travel insurance, while others just cover one specific area, so it’s best to check your benefits before making a claim.
For most claims, you’ll be required to provide documents to verify your claim, that could include:
Many credit cards also require you to file your claims within a certain window, which will typically range from 20 to 60 days. You can often file online via your credit card’s benefit provider website or you can call to get the process started.
For many occasions, yes, the travel insurance provided by your credit card will likely be enough. But there might be times in when the coverage doesn’t apply or it doesn’t meet all your needs.
While credit card travel insurance can come in handy in many ways, it’s important to plan ahead and know your card’s limitations, so you can have a backup where needed.
Yes, depending on the card you have, it could provide you with travel insurance. Travel insurance is a common benefit for premium cards that have annual fees. However, there are credit cards that offer these perks with no annual fee.
The best way to know if your credit card provides travel insurance is to check the card’s benefits guide. Make sure you fully understand your card’s benefits and when they will and won’t apply to your travel bookings.
The exact coverage varies, but a few common insurance benefits are trip cancellation and interruption, lost or damaged baggage, trip delay, and theft or damage to rental cars.
At CNBC Select, our mission is to provide our readers with high-quality service journalism and comprehensive consumer advice so they can make informed decisions with their money. Every credit card list is based on rigorous reporting by our team of expert writers and editors with extensive knowledge of credit card products. While CNBC Select earns a commission from affiliate partners on many offers and links, we create all our content without input from our commercial team or any outside third parties, and we pride ourselves on our journalistic standards and ethics. See our methodology for more information on how we choose the best credit cards for travel protection.
Subscribe to the CNBC Select Newsletter!
Money matters — so make the most of it. Get expert tips, strategies, news and everything else you need to maximize your money, right to your inbox. Sign up here.
To determine which cards offer the best travel protection, CNBC Select analyzed over 250 major credit cards issued through FDIC-insured banks and NCUA-insured credit unions that are widely available in the U.S.
We compared each card on a range of travel protection features, such as what types of trips are covered and what conditions have to be met for you to take advantage of the benefit. We also considered overall cost, factoring in annual fees, welcome bonuses, introductory and standard APRs, balance transfer fees and foreign transaction fees. We also considered the application process when available (e.g., is there a credit pull or required credit score).
We also considered CNBC Select audience data when available, such as general demographics and engagement with our content and tools.
Catch up on CNBC Select’s in-depth coverage of credit cards, banking and money, and follow us on TikTok, Facebook, Instagram and Twitter to stay up to date.
Editorial Note: Opinions, analyses, reviews or recommendations expressed in this article are those of the Select editorial staff’s alone, and have not been reviewed, approved or otherwise endorsed by any third party.
Treatment with belzutifan (Welireg) plus pembrolizumab (Keytruda) and lenvatinib (Lenvima) improved efficacy outcomes compared with multiple other pembrolizumab-based triplet regimens in patients with advanced clear cell renal cell carcinoma (ccRCC), according to findings from substudy 3A of the phase 1/2 KEYMAKER-U03 trial (NCT04626479) presented at the
Providing the background for the study, Cristina Suarez Rodriguez, MD, PhD, of Vall d’Hebron University Hospital, Barcelona, Spain, said, “First-line triplet regimens adding novel mechanisms of action to standard doublet therapy may be a promising approach for advanced clear cell renal cell carcinoma…The potent and selective HIF-2α inhibitor belzutifan is a treatment for advanced RCC following prior anti-PD-(L1) and VEGFR-TKI therapy, and may be a highly suitable candidate for novel triplet therapy in the first-line setting.”
For substudy 3A (NCT04626479) of the umbrella phase 1/2 KEYMAKER-U03 trial, investigators sought to evaluate novel pembrolizumab-based regimens as first-line treatment for advanced ccRCC.
“The study employed an adaptive design in which experimental arms were added and/or inactivated on a rolling basis with a continuous enrolling reference arm. Experimental arms each had a safety lead-in phase of 10 patients,” Suarez Rodriguez explained.
Patients with histologically confirmed locally advanced or metastatic ccRCC as measurable by RECIST v1.1, no prior systemic therapy for advanced RCC, and a KPS score of at least 70% were eligible for the study. Following the safety lead-in phase, the study entered the randomized phase. In this phase, patients were randomly assigned to 2:1 to 1 of 5 arms. Arm 1 included coformulated favezelimab/pembrolizumab 800mg/200 mg intravenously every 3 weeks, and lenvatinib 20 mg orally once daily. Arm 2 included coformulated vibostolimab/pembrolizumab 200/200 mg IV every 3 weeks plus belzutifan 120 mg orally once daily. Arm 3 included coformulated quavonlimab/pembrolizumab 25 mg/400 mg IV every 6 weeks plus lenvatinib 20 mg orally once daily. Arm 4 included belzutifan 120 mg orally once daily plus pembrolizumab 400 mg IV every 6 weeks, plus lenvatinib 20 mg orally once daily. A concurrent reference arm included pembrolizumab 400 IV every 6 weeks plus lenvatinib 20 mg orally once daily.
Primary end points were overall responsive rate (ORR) as measured by RECIST v1.1 by blinded independent central review (BICR), as well as safety. Secondary end points included duration of response (DOR) and progression-free survival by RECIST v1.1 by BICR, as well as overall survival (OS).
Suarez Rodriguez reported ORR for the arms as follows:
• Arm 1: At a median follow-up of 39.2 months (range, 28.8-44.6 months), ORR was 62.7% (95% CI: 48.1-75.9).
• Arm 2: At a median follow-up of 16.4 months (range, 11.8-23.4 months), ORR was 42.5% (95% CI: 31.5-54.1).
• Arm 3: At a median follow-up of 22.1 months (range, 13.5-40.6 months), ORR was 71.3% (95% CI: 60.0-80.8).
• Arm 4: At a median follow-up of 23.4 months (range, 14.1-41.0 months), ORR was 77.5% (95% CI: 66.8-86.1).
• Reference arm: At a median follow-up of 21.2 months (range, 11.9-44.4 months), ORR was 80.6% (95% CI: 68.6-89.6).
Complete response was observed in 5 (9.8%), 4 (5.0%), 5 (6.3%), 10 (12.5%), and 4 (6.5%) patients across the respective arms.
In arm 1, DOR and PFS were found to be numerically similar to the reference arm, Suarez Rodriguez reported. The treatment regimen of arm 2 was found to be not superior to the reference arm. For arm 3, median DOR was 25.0 months (range, 2.4-37.1+ months) in arm 3 and 25.6 months (range, 1.4+-27.6 months) in the reference arm.
Arm 4 “showed a median duration of response of 33 months vs 26 months in the reference arm,” Suarez Rodriguez said. Median PFS was 31.8 months (95% CI: 26.3-NR) in arm 4 vs 20.8 months (95% CI: 12.4-29.0) in the reference arm (HR, 0.45, 95% CI: 0.25-0.83). OS was not reached in either arm.
Suarez Rodriguez also discussed safety across the regimens. Arm 1 had the highest incidence of grade 3 or higher adverse events (AEs) (86.9%), followed by arm 3 (73.3%), the reference arm (71.0%), arm 4 (70.0%), and arm 2 (68.9%). Arm 1 had the highest rate of treatment-related AEs (37.7%), followed by arm 3 (34.4%), arm 2 (31.1%), the reference arm (30.6%), and arm 4 (24.4%). Serious treatment-related AEs were highest in arm 1 (36.1%) followed by arm 3 (34.4%), the reference arm (30.6%), arm 2 (27.8%), and arm 4 (22.2%).
“As expected, the most common adverse event observed in the combination arms containing lenvatinib was hypertension. The most common adverse event in the combinations containing belzutifan was anemia,” Suarez Rodriguez said.
“In conclusion, observed efficacy of pembrolizumab plus lenvatinib were confirmatory of prior observations for this combination. Belzutifan/pembrolizumab plus lenvatinib and the anti-CTLA-4 combination had similar overall response rate compared to pembrolizumab/lenvatinib as first-line therapy in patients with previously untreated advanced clear cell renal cell carcinoma. Responses were potentially less favorable in the anti-LAG-3 and anti-TIGIT arms. Belzutifan plus pembrolizumab plus lenvatinib, but not the other investigative arms, may have been associated with a higher proportion of complete response, prolonged duration of response, and prolonged progression-free survival compared to pembrolizumab plus lenvatinib,” Suarez Rodriguez said in her concluding remarks.
Suarez Rodriguez also noted that belzutifan plus pembrolizumab plus lenvatinib and quavonlimab/pembrolizumab plus pembrolizumab are being evaluated in the phase 3 LITESPARK-012 study (NCT047367706).
DISCLOSURES: Suarez Rodriguez noted research funding and medical writing support from Merck Sharp & Dohme (MSD) LLC; research funding from Bristol Myers Squibb, Ipsen, Pfizer, and Roche; advisory board membership with Astellas, AstraZeneca, Bristol Myers Squibb, and MSD; travel accommodations from Bayer, speakers’ bureau membership with AstraZeneca, Astellas, Bristol Myers Squibb, Ipsen, and MSD; an invited speaker for Bristol Myers Squibb and MSD; and expert testimony for Bristol Myers Squibb and MSD.
1. Suarez Rodriguez C, Rojas CI, Shin SJ, et al. First-line pembrolizumab-based regimens for advanced clear cell renal cell carcinoma: KEYMAKER-U03 substudy 03A. Presented at: European Society for Medical Oncology Congress. October 17-21, 2025. Berlin, Germany. Abstract LBA96. https://s3.eu-central-1.amazonaws.com/m-anage.com.storage.esmo/static/esmo2025_abstracts/LBA96.html.pdf

Researchers have engineered a courtship ritual from one species of fruit fly into another using genetic modification.
A Japanese research team tweaked a single gene in the fly Drosophila melanogaster, causing it to display a courtship ritual…

France’s Guillaume Cizeron is no stranger to feeling victorious on Grand Prix de France ice, having won this Grand Prix stage a record six times with his former partner Gabriella…
The combination of lenvatinib (Lenvima) and everolimus (Afinitor) reduced the risk of progression or death by 49% compared with cabozantinib (Cabometyx) for patients with metastatic clear cell renal cell carcinoma (ccRCC) following progression on a PD-1 inhibitor, according to findings from a phase 2 study presented at the
In the multicenter phase 2 study, the median progression-free survival (PFS), which was the primary end point of the study, was 15.7 months with the combination of lenvatinib plus everolimus compared with 10.2 months with cabozantinib (HR, 0.51; 95% CI, 0.29-0.89; P = .02). There were more adverse events (AEs) observed with the combination, but these differences were not deemed statistically significant. Grade 3/4 AEs were experienced by 67.5% of those treated with lenvatinib plus everolimus compared with 50% of those receiving cabozantinib (odds ratio [OR], 2.08; 95% CI, 0.86-5.02).
“Lenvatinib plus everolimus significantly prolonged progression-free survival over cabozantinib,” Andrew W. Hahn, MD, Department of Genitourinary Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, said during a presentation of the results. “As the first head-to-head randomized comparison of contemporary second-line or later treatments after immune checkpoint inhibition, these results are relevant to treatment sequencing and inform oncology practice.”
The study enrolled 86 patients, with 40 receiving the combination and 46 receiving cabozantinib. For the combination arm, lenvatinib was administered at 18 mg per day and everolimus was given at 5 mg per day. Cabozantinib was administered at 60 mg per day. The most common prior treatment was the combination of nivolumab (Opdivo) and ipilimumab (Yervoy), which was received by 70.9% of patients enrolled in the study. This was followed by the combination of pembrolizumab (Keytruda) and axitinib (Inlyta) for 16.3%, adjuvant pembrolizumab for 4.7%, and any other checkpoint inhibitor for 8.1%.
Baseline characteristics were well balanced between the groups with most patients (69.8%) in each group having received 1 prior line of therapy and 30.2% having received 2 prior lines of therapy. Nearly half of patients had received prior VEGF-targeted therapy (45% for the combination arm and 41.3% for the single agent group). The IMDC risk was primarily intermediate in both groups (80% for combination and 73.9% for single agent). The IMDC risk was poor for 7.5% of those in the combination arm and for 8.7% of those in the control arm.
The objective response rate was 52.6% with lenvatinib plus everolimus compared with 38.6% with cabozantinib. Although numerically higher, this rate did not pass the bar for statistical significance (OR, 1.87; 95% CI, 0.75-4.6; P = .17). In addition to responses, stable disease was observed in 39.5% of those receiving the combination and for 54.5% of those in the single-agent arm.
The study was not designed to assess differences in overall survival (OS) and findings for this end point were still immature, Hahn noted. After a median of 20 months of follow-up, there had been 11 events in the combination arm and 13 in the control group. The 1-year OS probability was 87.0% with lenvatinib plus everolimus compared with 84.6% with cabozantinib (HR, 1.05; 95% CI, 0.47-2.38; P = .86).
Serious AEs were experienced by 27.5% of those treated with lenvatinib plus everolimus compared with 19.6% of those in the cabozantinib arm (OR, 1.56; 95% CI, 0.57-4.24). Dose interruptions were necessary for 70% of those in the combination arm compared with 78.3% of those in the cabozantinib group (OR, 0.65; 0.24-1.73). Dose reductions were necessary for 57% and 60.9% of those in the combination and control arm, respectively (OR, 0.80; 95% CI, 0.33-1.93). Treatment discontinuation was more common in the combination group at 20% compared with the single agent at 10.9% (OR, 2.05; 95% CI, 0.61-6.91).
“Of the 8 patients who discontinued treatment with lenvatinib plus everolimus, 5 of those were due to proteinuria,” said Hahn.
The most commonly observed AEs in the study aligned with those historically associated with each agent, Hahn noted. For the combination and single agent arms, respectively, the most common all-grade AEs were diarrhea (70% vs 73.9%), fatigue (72.5% vs 60.9%), proteinuria (65% vs 37%), hypertension (57.5% vs 39.1%), nausea (40% vs 39.1%), palmar-plantar erythrodysesthesia (20% vs 52.2%), vomiting (32.5% vs 34.8%), and oral mucositis (15% vs 43.5%).
“This is a positive phase 2 trial and demonstrates improved PFS and response compared with cabozantinib, but with higher toxicity,” said invited discussant Lisa M. Pickering, consultant medical oncologist at The Royal Marsden, MD, PhD, FRCP. “So, is it positive enough? I think that depends on you and your patient, but I would say that we can and should consider using lenvatinib/everolimus, particularly in patients for whom the priority is response and to who the toxicity rate is acceptable.”
The combination of lenvatinib and everolimus was approved by the FDA in 2016 for the treatment of patients with advanced RCC after a prior anti-angiogenic agent.3 The phase 2 study was completed to assess the combination in a more contemporary treatment setting, Hahn noted.
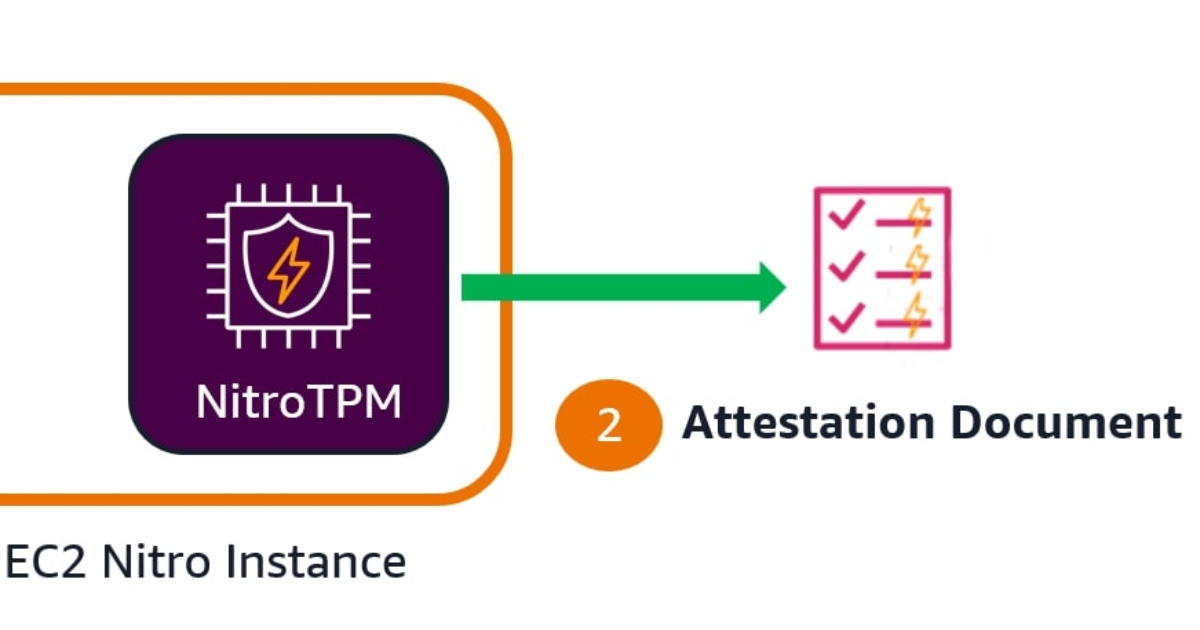
AWS has introduced EC2 instance attestation, a new security feature that enables customers to verify that their virtual machines are running approved software configurations in a cryptographically secure manner. The capability is powered…