
LAHORE, OCT 19 (APP/DNA): Governor Khyber Pakhtunkhwa Faisal Karim Kundi met with Federal Interior Minister Mohsin Naqvi at his residence in Lahore on Sunday to discuss the overall law and order situation in…


LAHORE, OCT 19 (APP/DNA): Governor Khyber Pakhtunkhwa Faisal Karim Kundi met with Federal Interior Minister Mohsin Naqvi at his residence in Lahore on Sunday to discuss the overall law and order situation in…

On October 21, 2025, the interstellar object 3I/ATLAS will be exactly on the opposite side of the Sun relative to Earth, constituting a so-called `solar-conjunction’. When you…
At the ESMO Congress 2025 in Berlin, Dr. Jean-Baptiste Bachet (Paris, France) presented the final results of the phase II OPTIPRIME trial (Abstract 727MO), a multicentre French study evaluating a novel “stop-and-go” strategy combining FOLFOX and panitumumab (Pmab) in patients with RAS/BRAF wild-type metastatic colorectal cancer (mCRC).
At OncoDaily GI, we spotlight the innovations reshaping colorectal cancer care — from adaptive treatment sequencing to intelligent use of targeted therapies to mitigate resistance and cumulative toxicity.
Epidermal growth factor receptor (EGFR) blockade has long been a cornerstone of therapy in RAS/BRAF wild-type mCRC, delivering significant response rates and survival benefits. Yet, the cumulative toxicities associated with prolonged anti-EGFR exposure — particularly dermatologic and nail toxicities — often limit treatment duration. Furthermore, continuous EGFR inhibition can foster resistant subclones, potentially diminishing long-term efficacy.
The OPTIPRIME trial was designed to test whether an intermittent (“stop-and-go”) use of panitumumab, alongside oxaliplatin-based chemotherapy, could maintain efficacy while improving tolerability and extending the duration of disease control (DDC) — a metric capturing both the initial and subsequent treatment phases under EGFR-targeted therapy.
Conducted across 36 French centers between April 2018 and May 2023, OPTIPRIME enrolled 118 patients (mITT: 115 after excluding 3 with RAS or BRAF mutations). Eligible patients had previously untreated, measurable, RAS/BRAF wild-type mCRC with ECOG performance status 0–1.
Treatment consisted of
The primary endpoint, DDC, was defined as the time from inclusion to radiological progression under EGFR therapy with chemotherapy or death. Patients undergoing R0/R1 metastasectomy were censored at surgery, and those switching therapy without progression were censored at the time of switch.
The study was powered to detect an improvement from a median DDC of 14 months (H0) to 20 months (H1), with a 1-sided α of 5% and 80% power. The predefined threshold for success (upper critical value) was 17.84 months.
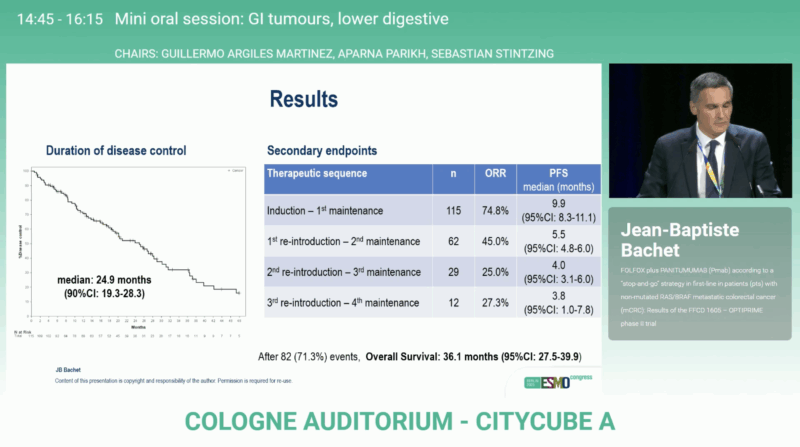
After a median follow-up of 47.9 months, OPTIPRIME achieved its primary endpoint with a median DDC of 24.9 months (90 % CI 19.3–28.3), surpassing the predefined efficacy boundary. The overall response rate reached 74.8 %, reflecting substantial activity of the FOLFOX + panitumumab regimen.
Most patients (83.5 %) entered at least one maintenance phase, and many underwent repeated reintroductions of panitumumab, illustrating the practical feasibility of this cyclic regimen. A subset (11 %) proceeded to metastasectomy with curative intent. At data cut-off, 69.6 % of patients had received subsequent second-line therapy.
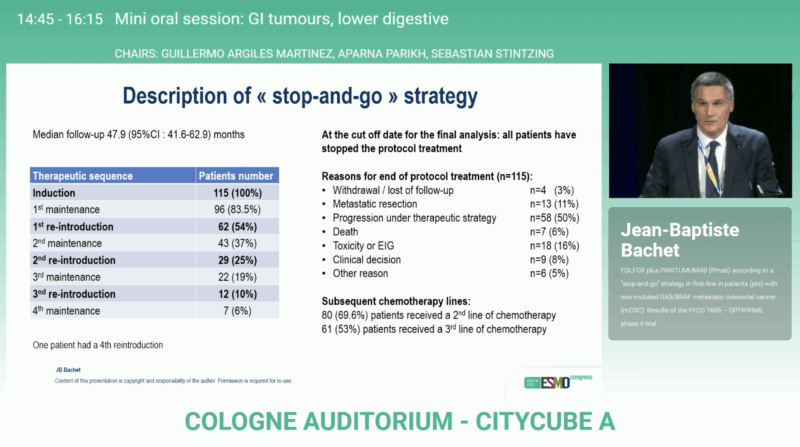
The median overall survival was 36.1 months (95 % CI 27.5–39.9), among the best reported for first-line mCRC in this molecular subgroup. Median progression-free survival (PFS) during the induction and first maintenance phase was 9.9 months (95% CI 8.3–11.1), confirming durable early disease control. These findings confirm that intermittent EGFR inhibition can sustain long-term disease control while preserving patient quality of life and delaying resistance.
The OPTIPRIME trial successfully met its primary endpoint, validating a biologically rational and clinically practical strategy for optimizing EGFR-targeted therapy in mCRC. The median DDC of nearly 25 months underscores the durability achievable through treatment holidays, allowing patients to recover from toxicity while preserving future sensitivity to anti-EGFR agents.
This “stop-and-go” model represents an important step forward in adaptive treatment design, addressing one of the key dilemmas in metastatic colorectal cancer: how to balance intensity, tolerance, and long-term benefit. It aligns with growing evidence from molecular studies showing that resistant EGFR clones can decay during drug-free intervals, restoring sensitivity upon rechallenge.
The OPTIPRIME findings strengthen the case for flexible, individualized sequencing of anti-EGFR therapy in mCRC. By alternating active and maintenance phases, clinicians can maintain high disease control rates while minimizing cumulative toxicity and optimizing quality of life.
With median survival exceeding three years, the study underscores how thoughtful integration of targeted agents within an adaptive framework can meaningfully extend outcomes in metastatic colorectal cancer.
You can read the full abstract here.
The OPTIPRIME phase II trial (NCT03584711) validates the clinical value of a “stop-and-go” panitumumab strategy in RAS/BRAF wild-type mCRC. By integrating periods of EGFR inhibition and maintenance fluoropyrimidine therapy, investigators achieved both prolonged disease control and strong overall survival, with improved tolerability.
These results may inform future treatment paradigms emphasizing adaptive, toxicity-conscious sequencing of targeted agents — a principle likely to gain further traction as precision oncology continues to evolve.

You can read about PERISCOPE II Trial: Randomized study finds no OS advantage for gastrectomy plus CRS/HIPEC versus systemic therapy on OncoDaily.

Governor Khyber Pakhtunkhwa Faisal Karim Kundi met with Federal Interior Minister Mohsin Naqvi at his residence in Lahore on Sunday to discuss the overall law and order situation in the province and recent incidents of terrorism.
During the…

Good morning, Aston Martin Aramco fans. This is your Captain speaking. Welcome to your special training detachment. You all know Formula One, but just how much do really know about the science of what a driver is subjected to in the cockpit?
This…
Infinatamab deruxtecan (I-DXd) demonstrated intracranial efficacy with acceptable safety in patients with extensive-stage small cell lung cancer (ES-SCLC) and baseline brain metastases, according to data from the primary analysis of the phase 2 IDeate-Lung01 trial (NCT05280470) presented during the
In those with baseline brain metastases (n = 65), the intracranial confirmed objective response rate (cORR) was 46.2% (95% CI, 33.7%-59.0%). Specifically, 30.8% of patients achieved a complete response (CR) as their best overall response, 15.4% experienced a partial response (PR), and 44.6% had stable disease (SD); 1.5% of patients experienced progressive disease (PD), and 7.7% were not evaluable (NE). The confirmed disease control rate (cDCR) was 90.8% (95% CI, 81.0%-96.5%). The median duration of response (DOR) was 6.2 months (95% CI, 4.0-7.9), and the median time to response (TTR) was 1.4 months (range, 0.9-8.5).
Moreover, in patients who had not previously received brain radiotherapy for baseline brain metastases (n = 26), I-DXd elicited an intracranial cORR of 57.7% (95% CI, 36.9%-76.6%). In those with baseline brain target lesions (n = 29), the intracranial cORR with the agent was 65.5% (95% CI, 45.7%-82.1%), and the central nervous system (CNS) cDCR was 96.6% (95% CI, 82.2%-99.9%).
“Intracranial efficacy with I-DXd [at] 12 mg/kg was promising, with 30.8% of patients achieving an intracranial CR, contributing to an intracranial cORR of 46.2% and DCR of 90.8%,” Pedro Simoes da Rocha, MD, PhD, said in a presentation of the data. Rocha is a medical oncologist at Vall d’Hebron University Hospital in Barcelona, Spain, and an International Association for the Study of Lung Cancer (IASLC) SCLC committee member.
The multicenter, randomized, open-label, phase 2 study included patients with histologically or cytologically documented ES-SCLC who were at least 18 years of age, had an ECOG performance status no higher than 1, and had previously received at least 1 but no more than 3 lines of platinum-based chemotherapy.2 Patients must have experienced radiologically documented disease progression on or after their most recent previous systemic treatment; they also needed to have at least 1 measurable lesion by RECIST 1.1 criteria. Those with asymptomatic brain metastases were allowed.
For part 1 of the study, the dose-optimization portion of the research, patients were randomly assigned 1:1 to receive I-DXd at 8 mg/kg every 3 weeks (n = 46; arm 1) or at 12 mg/kg every 3 weeks. For part 2, the extension portion, the agent was further examined at the 12-mg/kg dose.
The primary end point was ORR by blinded independent central review (BICR), and secondary end points included DOR, progression-free survival (PFS), DCR, and TTR by BICR and investigator assessment. Other end points comprised overall survival (OS), investigator-assessed ORR, safety, pharmacokinetics, and immunogenicity.
A subgroup analysis of patients with asymptomatic brain metastases identified by CNS BICR at study baseline was conducted and shared during the 2025 ESMO Congress.1 Brain CT or MRI was done at baseline for all patients. Those determined to have brain metastases had brain CT/MRI every 6 weeks for 36 weeks and every 12 weeks thereafter.
Of the 137 total patients who received I-DXd at a dose of 12 mg/kg, 65 had baseline brain metastases, and 72 did not. Of those who did, 39 received prior brain radiotherapy, and 26 did not. A total of 29 patients had brain target lesions at baseline with a median size of 17 mm (range, 10-68); 15 of these patients had prior brain radiotherapy and 14 did not.
The median patient age was 61.0 years (range, 39.0-76.0). Moreover, 80.0% of patients had an ECOG performance status of 1, and 20.0% had a status of 0. The median number of prior lines of systemic therapy received was 2 (range, 1-3).
In those with baseline brain metastases, the agent led to a systemic cORR of 46.2% (95% CI, 33.7%-59.0%). Best overall responses included CR (1.5%), PR (44.6%), and SD (43.1%); 7.7% of patients had PD, and 3.1% were NE. The systemic cDCR was 89.2% (95% CI, 79.1%-95.6%), the median DOR was 4.3 months (95% CI, 3.0-5.8), the median TTR was 1.4 months (range, 1.0-8.1), the median PFS was 4.5 months (95% CI, 4.0-5.4) and the median OS was 10.4 months (range, 7.9-15.3).
In those without baseline brain metastases (n = 72), the cORR with the agent was 50.0% (95% CI, 38.0%-62.0%) with best overall responses of CR in 2.8% of patients, PR in 47.2% of patients, and SD in 36.1% of patients; 6.9% of patients had PD, and 6.9% were NE. The cDCR in this group was 86.1% (95% CI, 75.9%-93.1%), the median DOR was 5.9 months (95% CI, 4.0-8.3), the median TTR was 1.4 months (range, 1.2-4.0), the median PFS was 5.4 months (95% CI, 4.2-6.7), and the median OS was 10.1 months (95% CI, 8.4-13.3).
Concordance between systemic and CNS objective response was 75.4%, Rocha said, adding that the concordance between systemic and CNS disease control was 86.2%. “OS and PFS were similar for patients with and without baseline brain metastases,” he said.
I-DXd showed intracranial efficacy irrespective of previous treatment for brain metastases at baseline. In those with prior radiotherapy (n = 39), the cORR was 38.5% (95% CI, 23.4%-55.4%); in those who received prior radiotherapy within 6 months prior to the study (n = 28), the cORR was 39.3% (95% CI, 21.5%-59.4%) and in those who received it 6 months or longer before study (n = 11), the cORR was 36.4% (95% CI, 10.9%-69.2%).
“Progression in the brain was uncommon, suggesting that I-DXd may prevent brain metastases,” Rocha added. Among the 65 patients with baseline brain metastases, 35.4% experienced progression in the brain; in those who had not received prior radiotherapy (n = 26), this rate was 23.1%, and in those who had (n =39), this rate was 43.6%. In those without baseline brain metastases (n = 72), 12.5% experienced progression in the brain.
The agent also elicited responses in those with brain target lesions at baseline (n = 29), he added. The CNS cORR in those without prior radiotherapy (n = 14) was 71.4% (95% CI, 41.9%-91.6%); in those with prior radiotherapy (n = 15), the CNS cORR was 60.0% (95% CI, 32.3%-83.7%). Concordance between systemic and CNS objective response was 69.0%, according to Rocha. The CNS DOR was 5.7 months (95% CI, 4.1-7.1) and the CNS TTR was 1.3 months (range, 0.9-3.0).
Any-grade treatment-related adverse effects (TRAEs) were experienced by 87.7% of patients with brain metastases at baseline (n = 65) and 91.7% of those without baseline brain metastases (n = 72); these effects were grade 3 or higher for 30.8% and 41.7% of patients, respectively. They were serious in 10.8% and 25.0% of cases. In those with baseline brain metastases, TRAEs led to dose delay, reduction, or treatment discontinuation for 23.1%, 15.4%, and 7.7% of patients; in those without baseline brain metastases, these rates were 27.8%, 15.3%, and 11.1%. TRAEs proved fatal for 1.5% and 6.9% of patients, respectively.
The most common TRAEs experienced by at least 10% of patients with baseline brain metastases were nausea (any grade, 49.2%; grade ≥3, 1.5%), decreased appetite (32.3%; 1.5%), neutropenia (30.8%; 6.2%), anemia (27.7%; 7.7%), asthenia (23.1%; 1.5%), fatigue (20.0%; 3.1%), lymphopenia (20.0%; 12.3%), diarrhea (16.9%; 0%), leukopenia (15.4%; 0%), thrombocytopenia (13.8%; 6.2%), increased aspartate aminotransferase level (10.8%; 1.5%), constipation (10.8%; 0%), and pneumonitis (10.8%; 0%).
The phase 3 IDeate-Lung02 study (NCT06203210) will be evaluating the intracranial activity of I-DXd vs physician’s choice of treatment in the form of topotecan, amrubicin, or lurbinectedin (Zepzelca) in patients with relapsed SCLC.3

 EPA
EPAPakistan and Afghanistan’s Taliban government have agreed to an “immediate ceasefire” after more than a week of deadly fighting.
The foreign ministry of Qatar,…
The past week has been a whirlwind of activity in the tech sector, with artificial intelligence (AI) taking center stage. From a $40 billion data center project to a new AI chip, the industry’s biggest players are making significant strides in…

I can’t tell you how many times I’ve needed files converted from one form to another. I might need to convert an image from .png to .jpg so it takes up less storage on my web hosting platform. I might need to convert a video from one…