Last night’s Dispatches was called Will AI Take My Job? Usually when something like this employs a question mark in the title, it’s because the answer is no. Not this time, though, because the sheer overwhelming inevitability of AI taking our…
Blog
-
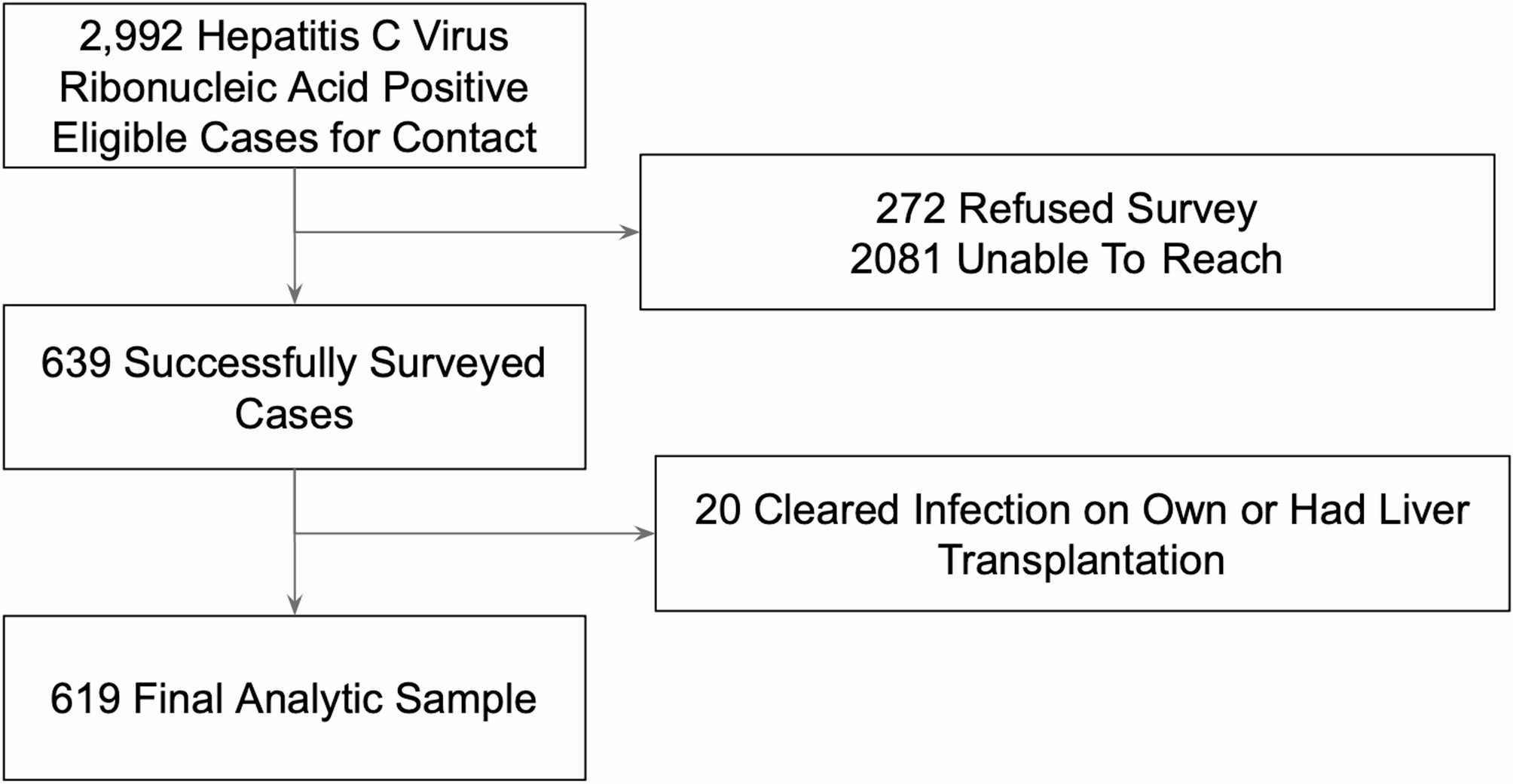
Determinants of hepatitis C virus treatment completion among Los Angeles County residents | BMC Infectious Diseases
We conducted a secondary data analysis of data collected in an ongoing linkage-to-cure project, Project Hepatitis C Virus Connect, a public-academic partnership between the Los Angeles County Department of Public Health and University of Southern…
Continue Reading
-
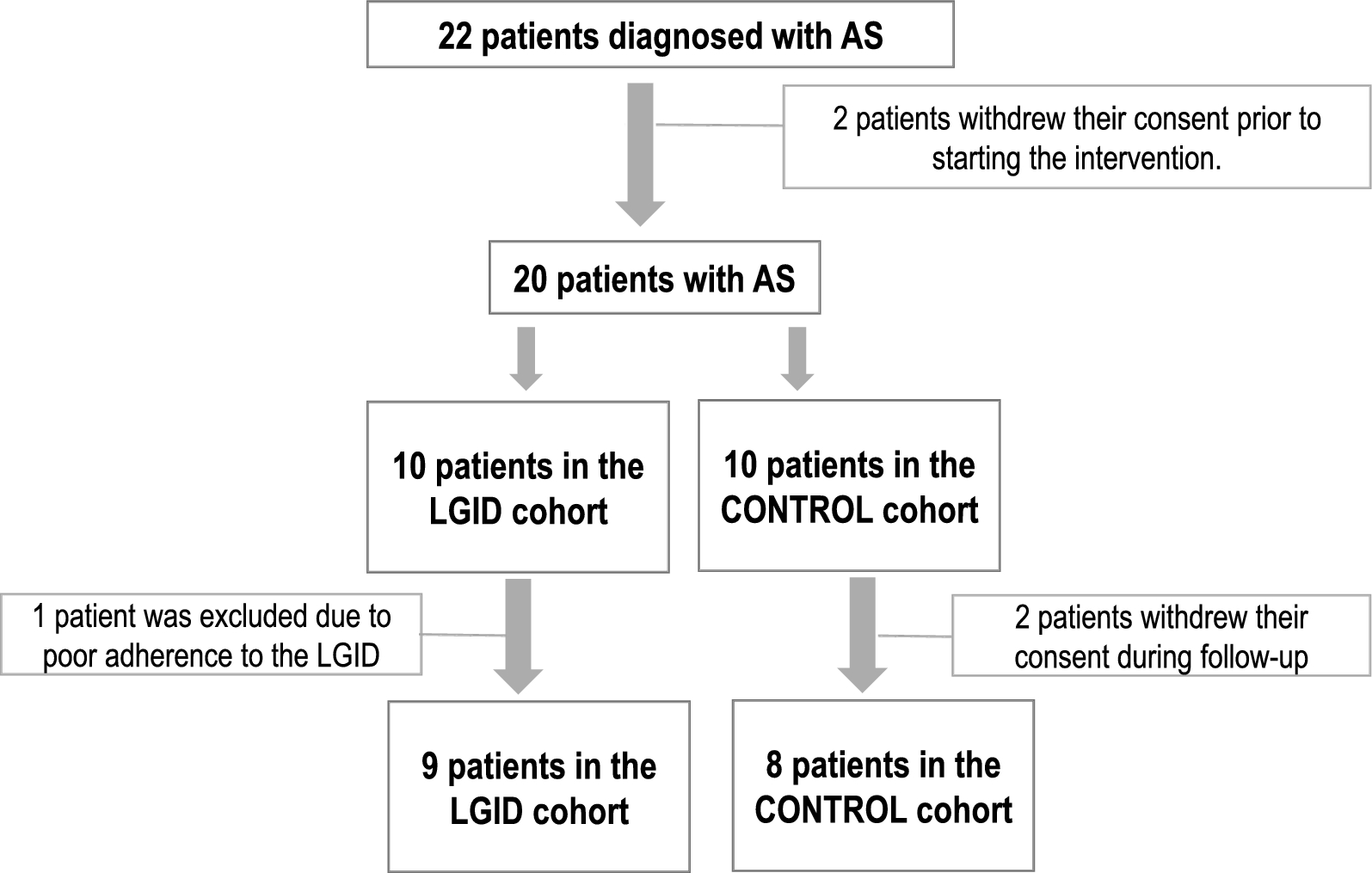
Efficacy and tolerability of a low-glycemic-index ketogenic diet in Angelman syndrome: findings from the DIANE study | Orphanet Journal of Rare Diseases
Our study, which included pediatric patients with Angelman Syndrome (AS), did not demonstrate that the low-glycemic-index diet (LGID) produces clinical improvement in any of the neurodevelopmental domains assessed using the Bayley Scales of Infant and Toddler Development-III.
Although various publications explore the use of the ketogenic diet (KD) in patients with AS, most of these studies focus on its efficacy for epilepsy control. However, there are few studies where the primary objective is to analyze the potential effects of KD on neurocognitive and behavioral development in this population. Previous research has reported that KD has positive cognitive and behavioral effects in pediatric patients with epilepsy, regardless of seizure control or the number of concomitant antiepileptic drugs [11, 15,16,17]. These benefits include improvements in alertness, attention, reciprocal social interaction, mood, sustained attention, receptive vocabulary, and information processing speed. Furthermore, various reviews have reported subjective data from parents describing their children as “more awake” and “more attentive” after starting the diet [17].
In the specific population with AS, Grocott et al. [18] retrospectively reviewed 23 patients treated with LGID and found that most achieved improved seizure control: 22% remained seizure-free, 43% experienced seizures only in specific contexts such as illness or non-convulsive status, and 30% showed a significant reduction in seizure frequency. Additionally, Thibert et al. [13] conducted a prospective study on the efficacy and tolerability of LGID in AS patients, reporting a reduction in seizure frequency in all patients, a reduction greater than 80% in five of them, and generalized improvements in EEG patterns. This study also reported a subjective perception of neurodevelopmental improvement from parents, although only some of these improvements were statistically significant in neuropsychological assessments.
Our results align with these findings. In our study, we observed qualitative improvement in the EEGs of patients treated with LGID compared to the habitual diet group after 24 weeks of intervention (44% improvement in the LGID group versus 25% in the habitual diet group). Moreover, in the LGID group, only 11% of patients experienced clusters of epileptic seizures, compared to 25% in the habitual diet group, which included one case of non-convulsive status epilepticus.
Although no statistically significant cognitive differences were achieved, a trend toward improvement was observed in receptive language, expressive language, and communication domains in the LGID group. Similarly, subjective parental perception reflected a global improvement in neurodevelopment in most cases. This outcome aligns with previous observations and reinforces the hypothesis that LGID may have neurocognitive benefits in patients with AS. In fact, five of the nine patients in the LGID group chose to continue the diet after completing the study, suggesting a positive impact perceived by families.
Sleep plays a fundamental role in the development and maintenance of memory and learning. Improving sleep quality and structure can significantly enhance sustained attention and memory in children. In patients with Angelman Syndrome (AS), it is estimated that approximately 80% experience moderate to severe sleep disturbances. Pelc et al. [26] conducted a clinical review in a small group of AS patients and described specific sleep characteristics, such as reduced total sleep duration, increased sleep-onset latency, altered sleep architecture, frequent nighttime awakenings, and reduced REM phase. Similarly, Spruyt et al. [27] conducted a systematic review and meta-analysis of 14 heterogeneous studies, mostly observational, and concluded that characteristic sleep problems in AS include reduced total sleep time, increased latency, frequent awakenings, and reduced sleep efficiency.
Additionally, Miano et al. [29] evaluated 10 children with AS using polysomnography and compared them with a control group of patients with intellectual disabilities with or without epilepsy. The results showed a significant increase in sleep state transitions, four times more frequent awakenings, and a 50% reduction in time spent in the deepest stage of sleep (NREM). This suggests considerably reduced sleep quality and lower sleep efficiency in AS patients.
Our results are consistent with these observations, as patients in our series showed reduced total sleep time, increased latency, frequent awakenings, and decreased sleep efficiency.
Pasca et al. [30] reviewed the effects of the ketogenic diet (KD) on sleep in neurological conditions such as autism spectrum disorders, epilepsy, and migraines, finding improvements in overall sleep quality, sleep-onset latency, reduction of nighttime awakenings, improved daytime sleepiness, and increased REM sleep. In our study, although a trend toward improvement in sleep quality was observed in the LGID group compared to the habitual diet group, these data were not statistically significant, preventing the assertion that LGID alone improves sleep structure.
It is important to note the limitations of this study. These include the small sample size, inherent to rare diseases such as Angelman syndrome, which limits statistical power. Additionally, the 24-week follow-up may have been insufficient to detect clinically meaningful changes in neurodevelopmental parameters, which often require longer durations to reach statistical significance. Developmental age equivalents were used as the primary outcome measure instead of more sensitive psychometric scores such as the Person Ability Score (PAS). Although this choice aligned with standard practice in 2021, it may have limited the detection of subtle changes in this population. Similarly, the use of standard scores from the VABS-II may have lacked sensitivity to small variations over time, particularly in individuals with profound impairment. As with the Bayley-III, this approach was consistent with prevailing clinical guidelines and research practices in Angelman syndrome; however, more sensitive metrics such as PAS could not be derived due to the unavailability of item-level conversion tools. Finally, some missing data resulted from incomplete caregiver diaries, likely due to the emotional and time burden of caregiving, as well as issues with the use of the Actiwatch by some children, which limits the reliability of the data.
In conclusion, although a trend toward greater evolutionary improvement was observed in the LGID group compared to the habitual diet group, these differences were not statistically significant in the various domains evaluated using the Bayley Scales of Infant and Toddler Development-III and cannot be solely attributed to the low-glycemic-index ketogenic diet. However, global improvements were identified in several variables evaluated at six months in the LGID group, and no serious adverse reactions attributable to the diet were reported.
These preliminary results do not support recommending the low-glycemic-index ketogenic diet as a generalized treatment for cognitive improvement in AS patients. Further studies with larger sample sizes and robust designs are needed to evaluate the potential impact of this intervention in this population.
Continue Reading
-

P8 Nico Hulkenberg was ‘determined to bounce back’ from Sprint ‘low blow’ in Austin
Nico Hulkenberg conceded that Sauber’s strong show of pace at the United States Grand Prix was “unexpected”.
Hulkenberg was the surprise story in Sprint Qualifying, as he positioned his Sauber in fourth place. But he was unable to translate…
Continue Reading
-

Ease.io Appoints Adam Wegel as CEO to Lead Next Phase of AI-Powered Manufacturing Innovation
SAN CLEMENTE, Calif., Oct. 21, 2025 /PRNewswire/ — Ease.io, a global leader in AI-powered software solutions that drive improvements in quality, safety, productivity, and compliance for manufacturers, today announced the…
Continue Reading
-

Giles introduces AI agent to accelerate healthcare and life science research
giles AI, a company making advanced research tools accessible to all and simplifying how scientists work with data, has announced the commercial launch of giles, its AI specialised research agent designed to support healthcare,…
Continue Reading
-
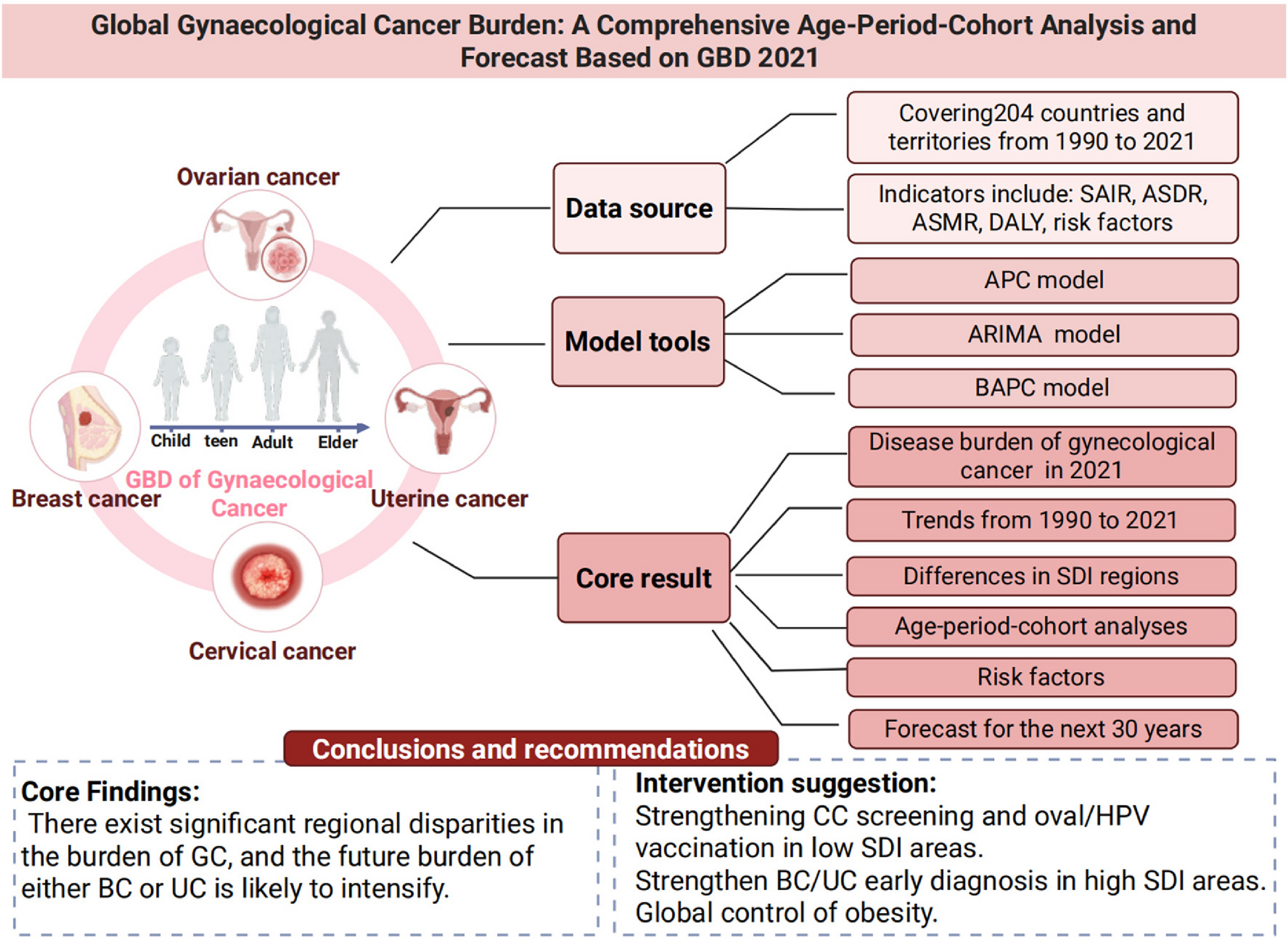
Trends and projections of global gynaecological cancer burden: age-period-cohort insights from Global Burden of Disease 2021 | BMC Women’s Health
Global and regional disease burden of GCs
The global burden of GCs exhibits significant variation across cancer types, regions, and socio-economic contexts. Among these, BC remains the most prevalent and deadliest, with continued increases in…
Continue Reading
-
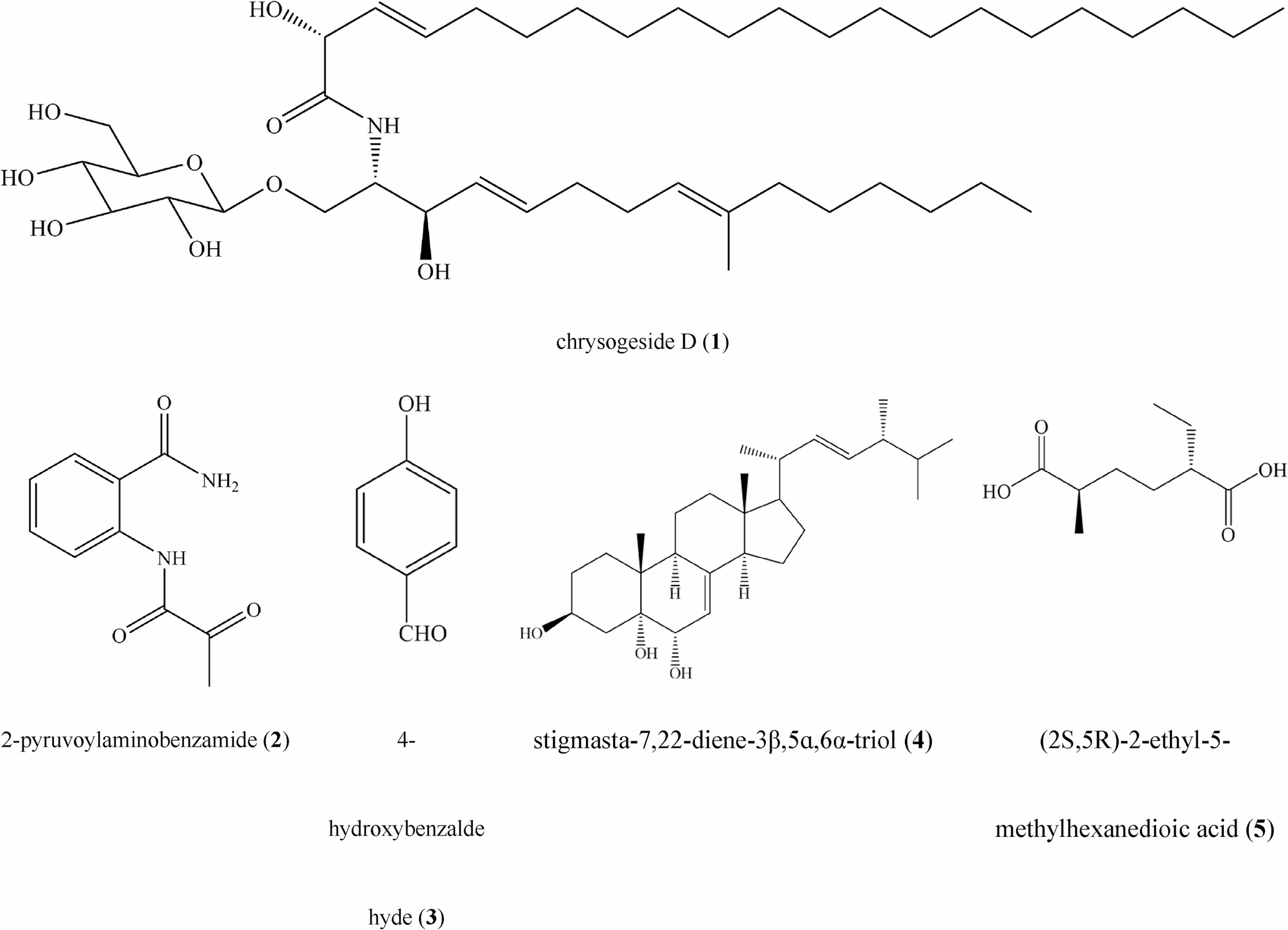
The effects of the secondary metabolites of the citrus endophytic fungus Nemania sp. LJZ-Y-11 on the citrus canker disease-causing pathogen Xanthomonas citri subsp. citri | BMC Microbiology
Reagents and equipment
Beef extract and bacteriological peptone purchased from Guangdong Huankai Microbial Sci. & Tech. Co., Ltd., dextrose purchased from Xilong Science Co., Ltd., and agar powder purchased from Beijing Solarbio Science &…
Continue Reading
-
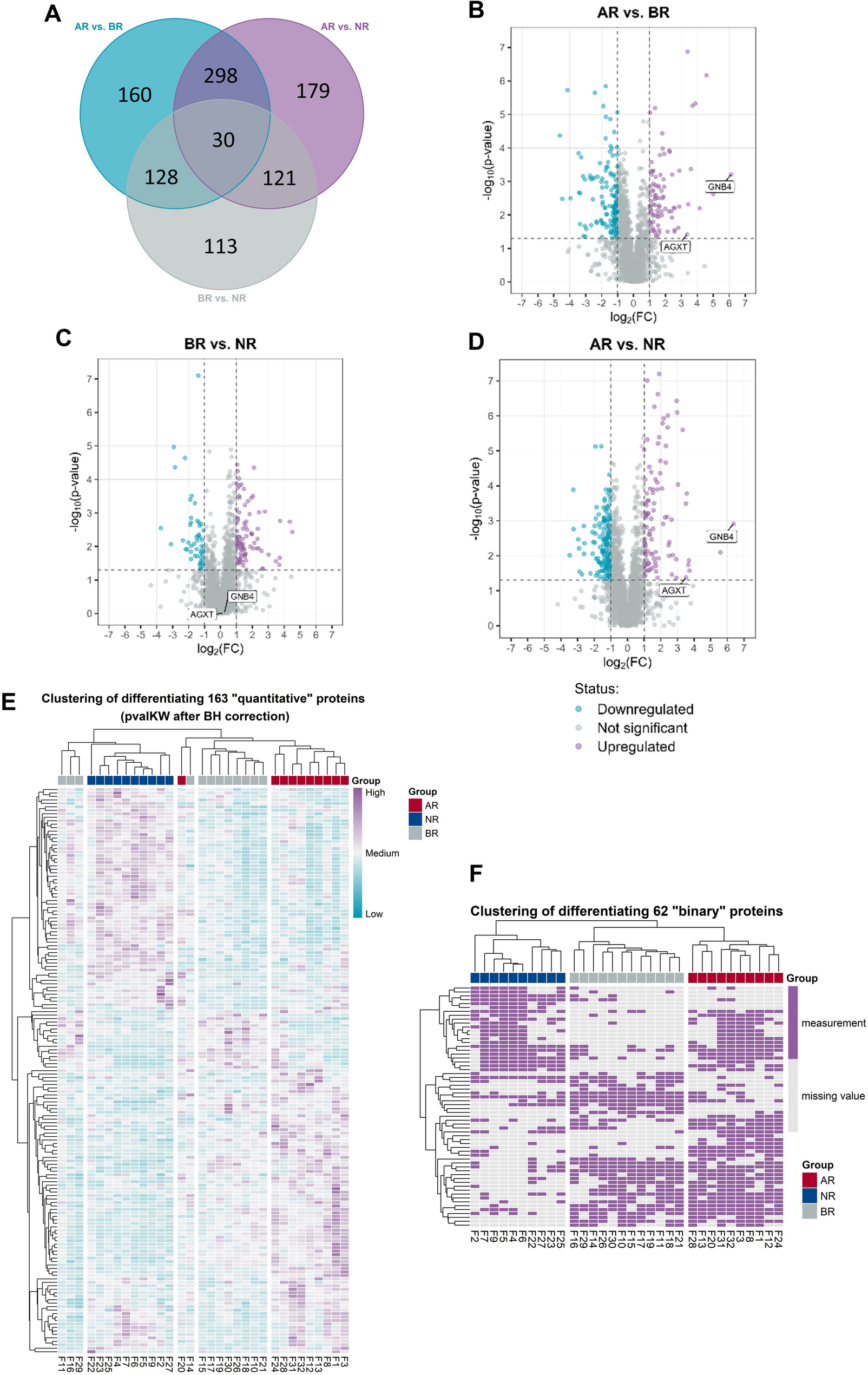
Biopsy proteome-based classification of T cell-mediated kidney allograft rejection | Journal of Translational Medicine
Starzl TE. History of clinical transplantation. World J Surg. 2000;24(7):759–82.
Google Scholar
Cooper JE. Evaluation and treatment of acute rejection in kidney allografts. Clin J Am Soc Nephrol CJASN. 2020;15(3):430–8.
Google Scholar
Lusco MA, Fogo AB, Najafian B, Alpers CE. AJKD atlas of renal pathology: acute T-cell–mediated rejection. Am J Kidney Dis. 2016;67(5):e29-30.
Google Scholar
Naesens M, Roufosse C, Haas M, Lefaucheur C, Mannon RB, Adam BA, et al. The Banff 2022 kidney meeting report: reappraisal of microvascular inflammation and the role of biopsy-based transcript diagnostics. Am J Transplant. 2024;24(3):338–49.
Google Scholar
Loupy A, Haas M, Roufosse C, Naesens M, Adam B, Afrouzian M, et al. The Banff 2019 kidney meeting report (I): updates on and clarification of criteria for T cell– and antibody-mediated rejection. Am J Transplant. 2020;20(9):2318–31.
Google Scholar
Nankivell BJ, Agrawal N, Sharma A, Taverniti A, P’Ng CH, Shingde M, et al. The clinical and pathological significance of borderline T cell–mediated rejection. Am J Transplant. 2019;19(5):1452–63.
Google Scholar
Loupy A, Mengel M, Haas M. Thirty years of the International banff classification for allograft pathology: the past, present, and future of kidney transplant diagnostics. Kidney Int. 2022;101(4):678–91.
Google Scholar
de Freitas DG, Sellarés J, Mengel M, Chang J, Hidalgo LG, Famulski KS, et al. The nature of biopsies with “borderline rejection” and prospects for eliminating this category. Am J Transplant. 2012;12(1):191–201.
Google Scholar
Mehta RB, Tandukar S, Jorgensen D, Randhawa P, Sood P, Puttarajappa C, et al. Early subclinical tubulitis and interstitial inflammation in kidney transplantation have adverse clinical implications. Kidney Int. 2020;98(2):436–47.
Google Scholar
Heilman RL, Nijim S, Chakkera HA, Devarapalli Y, Moss AA, Mulligan DC, et al. Impact of acute rejection on kidney allograft outcomes in recipients on rapid steroid withdrawal. J Transplant. 2011;2011(1):583981.
Google Scholar
Masin-Spasovska J, Spasovski G, Dzikova S, Petrusevska G, Dimova B, Lekovski L, et al. The evolution of untreated borderline and subclinical rejections at first month kidney allograft biopsy in comparison with histological changes at 6 months protocol biopsies. Prilozi. 2005;26(1):25–33.
Google Scholar
Thierry A, Thervet E, Vuiblet V, Goujon JM, Machet MC, Noel LH, et al. Long-term impact of subclinical inflammation diagnosed by protocol biopsy one year after renal transplantation. Am J Transplant. 2011;11(10):2153–61.
Google Scholar
Wang C, Feng G, Zhao J, Xu Y, Li Y, Wang L, et al. Screening of novel biomarkers for acute kidney transplant rejection using DIA-MS based proteomics. PROTEOMICS – Clinical Appl. 2024;18(3):2300047.
Heidari SS, Nafar M, Kalantari S, Tavilani H, Karimi J, Foster L, et al. Urinary epidermal growth factor is a novel biomarker for early diagnosis of antibody mediated kidney allograft rejection: A urinary proteomics analysis. J Proteomics. 2021;30(240):104208.
Gwinner W, Karch A, Braesen JH, Khalifa AA, Metzger J, Naesens M, et al. Noninvasive diagnosis of acute rejection in renal transplant patients using mass spectrometric analysis of urine samples: a multicenter diagnostic phase III trial. Transplant Direct. 2022;8(5):e1316.
Google Scholar
Geoui T, Urlaub H, Plessmann U, Porschewski P. Extraction of proteins from formalin-fixed, paraffin-embedded tissue using the qproteome extraction technique and preparation of tryptic peptides for liquid chromatography/mass spectrometry analysis. Current Protocls Mol Biol. 2010;90(1):10–27.
Wiśniewski JR, Gaugaz FZ. Fast and sensitive total protein and Peptide assays for proteomic analysis. Anal Chem. 2015;87(8):4110–6.
Google Scholar
Conover WJ. Practical nonparametric statistics. 3rd ed. New York: Wiley; 1998.
Jonckheere AR. A distribution-free k-sample test against ordered alternatives. Biometrika. 1954;41(1–2):133–45.
Pallant J. SPSS Survival Manual: A Step by Step Guide to Data Analysis Using SPSS for Windows. 4th ed. McGraw Hill Open University Press; 2010.
Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale; 1988.
Armitage P. Tests for Linear Trends in Proportions and Frequencies. Biometrics. 1955;11(3):375.
Fang F, Liu P, Song L, Wagner P, Bartlett D, Ma L, et al. Diagnosis of T-cell-mediated kidney rejection by biopsy-based proteomic biomarkers and machine learning. Front Immunol. 2023;6(14):1090373.
Hofstraat R, Marx K, Blatnik R, Claessen N, Chojnacka A, Peters-Sengers H, et al. Highly Repeatable Tissue Proteomics for Kidney Transplant Pathology: Technical and Biological Validation of Protein Analysis using LC-MS/MS. bioRxiv; 2024. p 2024.06.14.599091.
Schmouder RL, Kunkel SL. The cytokine response in renal allograft rejection. Nephrol Dial Transplant. 1995;10(supp1):36–43.
Google Scholar
De Serres SA, Mfarrej BG, Grafals M, Riella LV, Magee CN, Yeung MY, et al. Derivation and validation of a cytokine-based assay to screen for acute rejection in renal transplant recipients. Clin J Am Soc Nephrol. 2012;7(6):1018.
Google Scholar
Karczewski J, Karczewski M, Glyda M, Wiktorowicz K. Role of TH1/TH2 cytokines in kidney allograft rejection. Transplant Proc. 2008;40(10):3390–2.
Google Scholar
Biro M, Munoz MA, Weninger W. Targeting Rho-GTPases in immune cell migration and inflammation. Br J Pharmacol. 2014;171(24):5491–506.
Google Scholar
Matsuda J, Asano-Matsuda K, Kitzler TM, Takano T. Rho GTPase regulatory proteins in podocytes. Kidney Int. 2021;99(2):336–45.
Google Scholar
Kurian SM, Velazquez E, Thompson R, Whisenant T, Rose S, Riley N, et al. Orthogonal comparison of molecular signatures of kidney transplants with subclinical and clinical acute rejection: equivalent performance is agnostic to both technology and platform. Am J Transplant. 2017;17(8):2103–16.
Google Scholar
Kurian SM, Williams AN, Gelbart T, Campbell D, Mondala TS, Head SR, et al. Molecular classifiers for acute kidney transplant rejection in peripheral blood by whole genome gene expression profiling. Am J Transplant. 2014;14(5):1164–72.
Google Scholar
Zhang M, Chen F, Feng S, Liu X, Wang Z, Shen N, et al. FBLN5 as one presumably prognostic gene potentially modulating tumor immune microenvironment for renal clear cell carcinoma in children and young adults. Pharmacogenomics Pers Med. 2024;19(17):27–40.
Huang W, Shi H, Hou Q, Mo Z, Xie X. GSTM1 and GSTT1 polymorphisms contribute to renal cell carcinoma risk: evidence from an updated meta-analysis. Sci Rep. 2015;5(1):17971.
Google Scholar
Li P, Li D, Lu Y, Pan S, Cheng F, Li S, et al. GSTT1/GSTM1 deficiency aggravated cisplatin-induced acute kidney injury via ROS-triggered ferroptosis. Front Immunol. 2024;25(15):1457230.
Beothe T, Docs J, Kovacs G, Peterfi L. Increased level of TXNIP and nuclear translocation of TXN is associated with end stage renal disease and development of multiplex renal tumours. BMC Nephrol. 2024;17(25):227.
Luczak M, Formanowicz D, Marczak Ł, Pawliczak E, Wanic-Kossowska M, Figlerowicz M, et al. Deeper insight into chronic kidney disease-related atherosclerosis: comparative proteomic studies of blood plasma using 2DE and mass spectrometry. J Transl Med. 2015;13(1):20.
Google Scholar
Abdullah EL, Jalalonmuhali M, Ng KP, Jamaluddin FA, Lim SK. The role of lymphocyte subset in predicting allograft rejections in kidney transplant recipients. Transplant Proceed. 2022;54(2):312–9.
Halloran PF. T cell-mediated rejection of kidney transplants: a personal viewpoint. Am J Transplant. 2010;10(5):1126–34.
Google Scholar
Liu B, Chen L, Huang H, Huang H, Jin H, Fu C. Prognostic and immunological value of GNB4 in gastric cancer by analyzing TCGA database. Dis Markers. 2022;16(2022):1–16.
Zhan J, Huang L, Niu L, Lu W, Sun C, Liu S, et al. Regulation of CD73 on NAD metabolism: unravelling the interplay between tumour immunity and tumour metabolism. Cell Commun Signal. 2024;22(1):387.
Google Scholar
Chen S, Wainwright DA, Wu JD, Wan Y, Matei DE, Zhang Y, et al. Cd73: an emerging checkpoint for cancer immunotherapy. Immunotherapy. 2019;11(11):983–97.
Google Scholar
Antonioli L, Pacher P, Vizi ES, Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19(6):355–67.
Google Scholar
Schneider E, Winzer R, Rissiek A, Ricklefs I, Meyer-Schwesinger C, Ricklefs FL, et al. CD73-mediated adenosine production by CD8 T cell-derived extracellular vesicles constitutes an intrinsic mechanism of immune suppression. Nat Commun. 2021;12(1):5911.
Google Scholar
Deutsch EW, Bandeira N, Perez-Riverol Y, Sharma V, Carver JJ, Mendoza L, et al. The proteomexchange consortium at 10 years: 2023 update. Nucleic Acids Res. 2023;51(D1):D1539–48.
Google Scholar
Perez-Riverol Y, Bandla C, Kundu DJ, Kamatchinathan S, Bai J, Hewapathirana S, et al. The PRIDE database at 20 years: 2025 update. Nucleic Acids Res. 2025;53(D1):D543–53.
Google Scholar
Continue Reading

