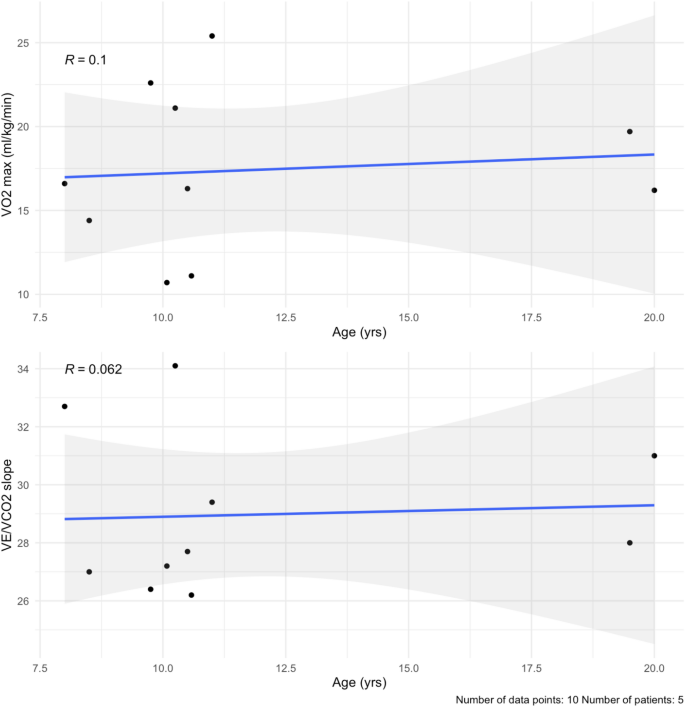
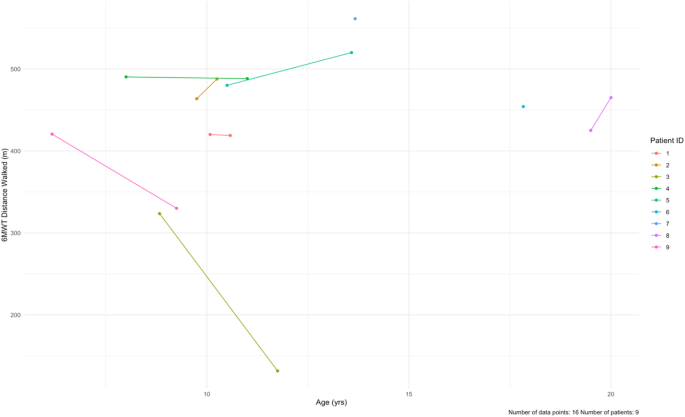
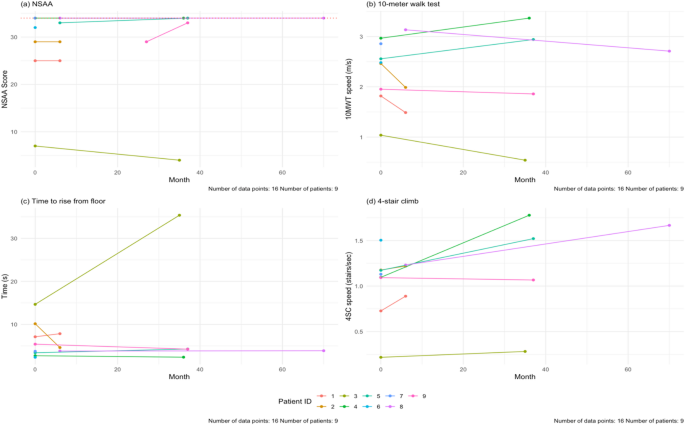
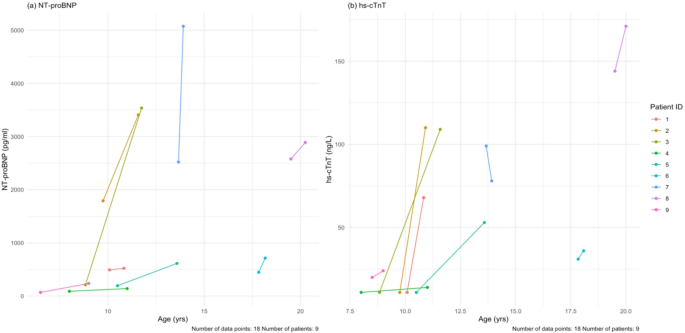
Dobson R, Giovannoni G. Multiple sclerosis – a review. Eur J Neurol. 2019;26(1):27–40. https://doi.org/10.1111/ene.13819.
Google Scholar
Lanz TV, Brewer RC, Ho PP, Moon JS, Jude KM, Fernandez D, et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and glialcam. Nature. 2022;603(7900):321–7. https://doi.org/10.1038/s41586-022-04432-7.
Google Scholar
Bjornevik K, Münz C, Cohen JI, Ascherio A. Epstein–Barr virus as a leading cause of multiple sclerosis: mechanisms and implications. Nat Rev Neurol. 2023;19(3):160–71. https://doi.org/10.1038/s41582-023-00775-5.
Google Scholar
Morandi E, Tanasescu R, Tarlinton RE, Constantinescu CS, Zhang W, Tench C, et al. The association between human endogenous retroviruses and multiple sclerosis: A systematic review and meta-analysis. PLoS ONE. 2017;12(2):e0172415. https://doi.org/10.1371/journal.pone.
Google Scholar
Küry P, Nath A, Créange A, Dolei A, Marche P, Gold J, et al. Human endogenous retroviruses in neurological diseases. Trends Mol Med. 2018;24(4):379–94. https://doi.org/10.1016/j.molmed.2018.02.007.
Google Scholar
Nevalainen T, Autio-Kimura A, Hurme M. Human endogenous retrovirus W in multiple sclerosis: transcriptional activity is associated with decline in oligodendrocyte proportions in the white matter of the brain. J Neurovirol. 2024;30(4):393–405. https://doi.org/10.1007/s13365-024-01208-9.
Google Scholar
Gruchot J, Reiche L, Werner L, Herrero F, Schira-Heinen J, Meyer U, et al. Molecular dissection of HERV-W dependent microglial- and astroglial cell polarization. Microbes Infect. 2025;27(5–6):105382. https://doi.org/10.1016/j.micinf.2024.
Google Scholar
Kremer D, Gruchot J, Weyers V, Oldemeier L, Gottle P, Healy L, et al. PHerv-w envelope protein fuels microglial cell-dependent damage of myelinated axons in multiple sclerosis. Proc Natl Acad Sci U S A. 2019;116(30):15216–25. https://doi.org/10.1073/pnas.1901283116.
Google Scholar
Gruchot J, Lewen I, Dietrich M, Reiche L, Sindi M, Hecker C, et al. Transgenic expression of the HERV-W envelope protein leads to polarized glial cell populations and a neurodegenerative environment. Proc Natl Acad Sci U S A. 2023;120(38):e2308187120. https://doi.org/10.1073/pnas.
Google Scholar
Charvet B, Pierquin J, Brunel J, Gorter R, Quetard C, Horvat B, et al. Human endogenous retrovirus type W envelope from multiple sclerosis demyelinating lesions shows unique solubility and antigenic characteristics. Virol Sin. 2021;36(5):1006–26. 10.7/s12250-021-00372-0.
Google Scholar
Perron H, Germi R, Bernard C, Garcia-Montojo M, Deluen C, Farinelli L, et al. Human endogenous retrovirus type W envelope expression in blood and brain cells provides new insights into multiple sclerosis disease. Mult Scler. 2012;18(12):1721–36. 10.177/1352458512441381.
Google Scholar
Ruberto S, Domınguez-Mozo MI, Garcıa-Martınez MA, Cossu D, Sechi LA, Alvarez-Lafuente R. Immune response profiling of HERV-W envelope proteins in multiple sclerosis: potential biomarkers for disease progression. Front Immunol. 2025;15:1505239. https://doi.org/10.3389/fimmu.2024.
Google Scholar
Mameli G, Poddighe L, Mei A, Uleri E, Sotgiu S, Serra C, et al. Expression and activation by Epstein Barr virus of human endogenous retroviruses-W in blood cells and astrocytes: inference for multiple sclerosis. PLoS ONE. 2012;7(9):e44991. https://doi.org/10.1371/journal.pone.0044991.
Google Scholar
Mameli G, Madeddu G, Mei A, Uleri E, Poddighe L, Delogu LG, et al. Activation of MSRV-type endogenous retroviruses during infectious mononucleosis and Epstein-Barr virus latency: the missing link with multiple sclerosis? PLoS ONE. 2013;8(11):e78474. https://doi.org/10.1371/journal.pone.0078474.
Google Scholar
Ebrahimkhani S, Vafaee F, Young PE, Hur SSJ, Hawke S, Devenney E, et al. Exosomal microrna signatures in multiple sclerosis reflect disease status. Sci Rep. 2017;7(1):14293. https://doi.org/10.1038/s41598-017-14301-3.
Google Scholar
Palazzo C, Asci I, Russo S, Buccoliero C, Mangialardi V, Abbrescia P, et al. Circulating exosomes with unique lipid signature in relapsing remitting multiple sclerosis. Front Cell Neurosci. 2025;19:1613618. https://doi.org/10.3389/fncel.2025.
Google Scholar
D’Anca M, Fenoglio C, Buccellato FR, Visconte C, Galimberti D, Scarpini E. Extracellular vesicles in multiple sclerosis: role in the pathogenesis and potential usefulness as biomarkers and therapeutic tools. Cells. 2021;10(7):1733. https://doi.org/10.3390/cells10071733.
Google Scholar
Teow SY, Liew K, Khoo AS, Peh SC. Pathogenic role of exosomes in Epstein-Barr virus (EBV)-associated cancers. Int J Biol Sci. 2017;13(10):1276–86. https://doi.org/10.7150/ijbs.19531.
Google Scholar
Meier UC, Cipian RC, Karimi A, Ramasamy R, Middeldorp JM. Cumulative roles for Epstein-Barr virus, human endogenous retroviruses, and human herpes virus-6 in driving an inflammatory cascade underlying MS pathogenesis. Front Immunol. 2021;12:757302. https://doi.org/10.3389/fimmu.2021.
Google Scholar
Mrad MF, Saba ES, Nakib L, Khoury SJ. Exosomes from subjects with multiple sclerosis express EBV-derived proteins and activate monocyte-derived macrophages. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e1004. 10.212/NXI.0000000000001004.
Google Scholar
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–73. https://doi.org/10.1016/S474-4422(17)30470-2.
Google Scholar
Kremer D, Forster M, Schichel T, Gottle P, Hartung HP, Perron H, et al. The neutralizing antibody GNbAC1 abrogates HERV-W envelope protein-mediated oligodendroglial maturation Blockade. Mult Scler. 2015;21(9):1200–3. 10.177/1352458514560926.
Google Scholar
Hartung HP, Derfuss T, Cree BA, Sormani MP, Selmaj K, Stutters J, et al. Efficacy and safety of Temelimab in multiple sclerosis: results of a randomized phase 2b and extension study. Mult Scler. 2022;28(3):429–40. https://doi.org/10.1177/13524585211024997.
Google Scholar
Curtin F, Champion B, Davoren P, Duke S, Ekinci EI, Gilfillan C, et al. A safety and pharmacodynamics study of temelimab, an antipathogenic human endogenous retrovirus type W envelope monoclonal antibody, in patients with type 1 diabetes. Diabetes Obes Metab. 2020;22(7):1111–21. 10./dom.14010.
Google Scholar
Simula ER, Jasemi S, Cossu D, Fais M, Cossu I, Chessa V, et al. Human endogenous retroviruses as novel therapeutic targets in neurodegenerative disorders. Vaccines. 2025;13(4):415. https://doi.org/10.3390/vaccines13040415.
Google Scholar
Chen J, Foroozesh M, Qin Z. Transactivation of human endogenous retroviruses by tumor viruses and their functions in virus-associated malignancies. Oncogenesis. 2019;8(1):6. https://doi.org/10.1038/s41389-018-0114-y.
Google Scholar
Brütting C, Stangl G, Staege M, Vitamin D. Epstein-Barr virus, and endogenous retroviruses in multiple sclerosis – facts and hypotheses. J Integr Neurosci. 2021;20(1):233–8. https://doi.org/10.31083/j.jin.2021.01.392.
Google Scholar
Latifi T, Zebardast A, Marashi SM. The role of human endogenous retroviruses (HERVs) in multiple sclerosis and the plausible interplay between HERVs, Epstein-Barr virus infection, and vitamin D. Mult Scler Relat Disord. 2022;57:103318. https://doi.org/10.1016/j.msard.2021.
Google Scholar
Gottle P, Schichel K, Reiche L, Werner L, Zink A, Prigione A, et al. TLR4 associated signaling disrupters as a new means to overcome HERV-W envelope-mediated myelination deficits. Front Cell Neurosci. 2021;15:777542. https://doi.org/10.3389/fncel.2021.
Google Scholar
Osaid Z, Haider M, Hamoudi R, Harati R. Exosomes interactions with the blood–brain barrier: implications for cerebral disorders and therapeutics. Int J Mol Sci. 2023;24(21):15635. https://doi.org/10.3390/ijms242115635.
Google Scholar
Gimenez-Orenga K, Oltra E. Human endogenous retrovirus as therapeutic targets in neurologic disease. Pharmaceuticals (Basel). 2021;14(6):495. https://doi.org/10.3390/ph14060495.
Google Scholar
Adler GL, Le K, Fu Y, Kim WS. Human endogenous retroviruses in neurodegenerative diseases. Genes. 2024;15(6):745. https://doi.org/10.3390/genes15060745.
Google Scholar
Bhetariya PJ, Kriesel JD, Fischer KF. Analysis of human endogenous retrovirus expression in multiple sclerosis plaques. J Emerg Dis Virol. 2017;3(2). https://doi.org/10.16966/2473-1846.133.
Moyes DL, Goris A, Ban M, Compston A, Griffiths DJ, Sawcer S, et al. HERV-K113 is not associated with multiple sclerosis in a large family-based study. AIDS Res Hum Retroviruses. 2008;24(3):363–5. https://doi.org/10.1089/aid.2007.0196.
Google Scholar
Brudek T, Christensen T, Aagaard L, Petersen T, Hansen HJ, Møller-Larsen A. B cells and monocytes from patients with active multiple sclerosis exhibit increased surface expression of both HERV-H Env and HERV-W Env, accompanied by increased seroreactivity. Retrovirology. 2009;6:104. https://doi.org/10.1186/742-4690-6-104.
Google Scholar
Laska MJ, Brudek T, Nissen KK, Christensen T, Moller-Larsen A, Petersen T, et al. Expression of HERV-Fc1, a human endogenous retrovirus, is increased in patients with active multiple sclerosis. J Virol. 2012;86(7):3713–22. https://doi.org/10.1128/JVI.06723-11.
Google Scholar
Censi ST, Mariani-Costantini R, Granzotto A, Tomassini V, Sensi SL. Endogenous retroviruses in multiple sclerosis: A network-based etiopathogenic model. Ageing Res Rev. 2024;99:102392. https://doi.org/10.1016/j.arr.2024.
Google Scholar
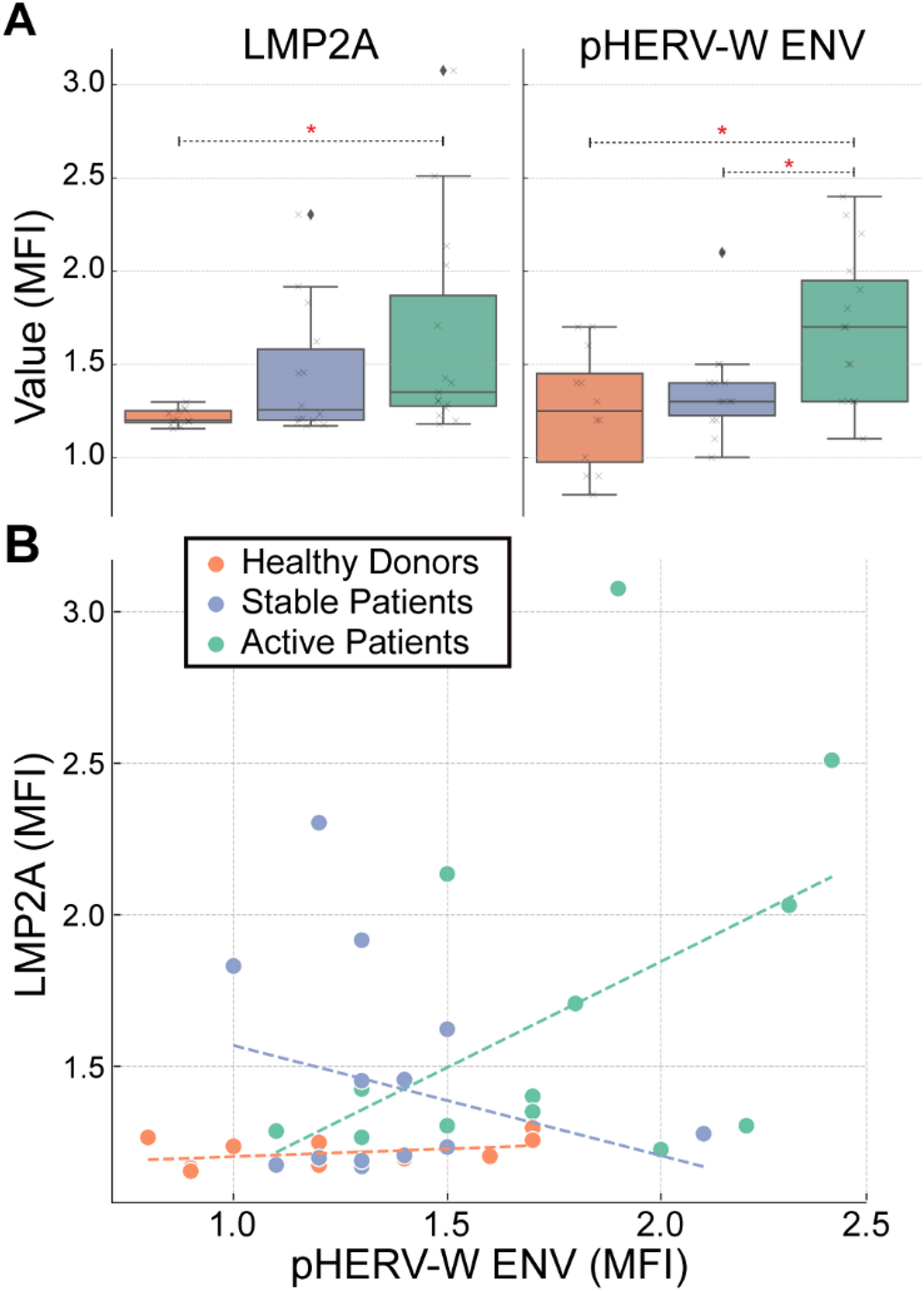
Ruberto S, Cossu D, Sechi LA. Correlation between antibodies against the pathogenic pHERV-W envelope protein and the inflammatory phase of multiple sclerosis. Immunology. 2024;171(2):270–6. https://doi.org/10.1111/imm.13712.
Google Scholar
Lyu L, Li Q, Wang C. EBV latency programs: molecular and epigenetic regulation and its role in disease pathogenesis. J Med Virol. 2025;97(7):e70501. https://doi.org/10.1002/jmv.
Google Scholar
Aarts SABM, Seijkens TTP, Dorst KJFv, Dijkstra CD, Kooij G, Lutgens E. The CD40–CD40L dyad in experimental autoimmune encephalomyelitis and multiple sclerosis. Front Immunol. 2017;8:1791. https://doi.org/10.3389/fimmu.2017.01791.
Google Scholar
Vermersch P, Wagner D, Mars LT, Noelle R, Giovannoni G. Inhibiting CD40 ligand in multiple sclerosis: a review of emerging therapeutic potential. Curr Treat Options Neurol. 2025;27:7. https://doi.org/10.1007/s11940-024-00818-2.
Google Scholar
Orian JM, D’Souza CS, Kocovski P, Krippner G, Hale MW, Wang X, et al. Platelets in multiple sclerosis: early and central mediators of inflammation and neurodegeneration and attractive targets for molecular imaging and site-directed therapy. Front Immunol. 2021;12:620963. https://doi.org/10.3389/fimmu.2021.
Google Scholar
Burnouf T, Walker TL. The multifaceted role of platelets in mediating brain function. Blood. 2022;140(8):815–27. https://doi.org/10.1182/blood.2022015970.
Google Scholar
Brudek T, Christensen T, Petersen T, Møller-Larsen A. Expression of HERV-H/W Env epitopes on PBMCs from MS patients with active disease. Retrovirology. 2011;8(Suppl 1):A210. https://doi.org/10.1186/742-4690-8-S1-A210.
Google Scholar
Chunder R, Schropp V, Kuerten S. B cells in multiple sclerosis and virus-induced neuroinflammation. Front Neurol. 2020;11:591894. https://doi.org/10.3389/fneur.2020.
Google Scholar
Garcia-Montojo M, Rodríguez-Martín E, Ramos-Mozo P, Ortega-Madueño I, Domínguez-Mozo M, Arias-Leal A, et al. Syncytin‐1/HERV‐W envelope is an early activation marker of leukocytes and is upregulated in multiple sclerosis patients. Eur J Immunol. 2020;50(5):685–94. https://doi.org/10.1002/eji.201948423.
Google Scholar
Banks WA, Sharma P, Bullock KM, Hansen KM, Ludwig N, Whiteside TL. Transport of extracellular vesicles across the Blood-brain barrier: brain pharmacokinetics and effects of inflammation. Int J Mol Sci. 2020;21(12):4407. https://doi.org/10.3390/ijms21124407.
Google Scholar
Gjelstrup MC, Stilund M, Petersen T, Møller H, Petersen EL, Christensen T. Subsets of activated monocytes and markers of inflammation in incipient and progressed multiple sclerosis. Immunol Cell Biol. 2017;96(2):160–74. https://doi.org/10.1111/imcb.025.
Google Scholar
Ferreira-Atuesta C, Reyes S, Giovanonni G, Gnanapavan S. The evolution of neurofilament light chain in multiple sclerosis. Front Neurosci. 2021;15:642384. https://doi.org/10.3389/fnins.2021.
Google Scholar
Virata MCA, Catahay JA, Lippi G, Henry BM. Neurofilament light chain: a biomarker at the crossroads of clarity and confusion for gene-directed therapies. Neurodegener Dis Manag. 2024;14(6):227–39. https://doi.org/10.1080/17582024.2024.2421738.
Google Scholar
Steffen F, Uphaus T, Ripfel N, Fleischer V, Schraad M, Gonzalez-Escamilla G, et al. Serum neurofilament identifies patients with multiple sclerosis with severe focal axonal damage in a 6-year longitudinal cohort. Neurol Neuroimmunol Neuroinflamm. 2023;10(1):e200055. https://doi.org/10.1212/NXI.0000000000200055.
Google Scholar
Freedman MS, Abdelhak A, Bhutani MK, Freeman J, Gnanapavan S, Hussain S, et al. The role of serum neurofilament light (sNfL) as a biomarker in multiple sclerosis: insights from a systematic review. J Neurol. 2025;272(6):400. https://doi.org/10.1007/s00415-025-13093-1.
Google Scholar