FULL TERMS AND CONDITIONS
PIXEL SUPERFANS ‘Trusted Tester Program’ OFFICIAL RULES (“Official Rules”) – US VERSION
NO PURCHASE NECESSARY. A PURCHASE WILL NOT INCREASE YOUR CHANCES OF WINNING.
ENTRY IN THIS CONTEST CONSTITUTES YOUR ACCEPTANCE…

FULL TERMS AND CONDITIONS
PIXEL SUPERFANS ‘Trusted Tester Program’ OFFICIAL RULES (“Official Rules”) – US VERSION
NO PURCHASE NECESSARY. A PURCHASE WILL NOT INCREASE YOUR CHANCES OF WINNING.
ENTRY IN THIS CONTEST CONSTITUTES YOUR ACCEPTANCE…

People with obesity who also have high muscle mass may be less likely to have obesity-related organ damage. This observation was described in the study “Handgrip Strength and Trajectories of Preclinical Obesity Progression: A…
The CD19/CD22-directed bispecific CAR T-cell therapy CAR2219 induced high response rates, including complete responses (CRs), in patients with relapsed/refractory large B-cell lymphoma (LBCL), according to findings from a prospective, single-arm, single-center, phase 2 trial (NCT06081478) presented at the
At a median follow-up of 4.2 months after CAR2219 infusion, among 31 treated patients, the best overall response rate (ORR) was 100%. The CR rate was 67.7% (95% CI, 48.6%-83.3%), and the partial response (PR) rate was 32.3%. Early responses were reported; at 4 weeks post-infusion, the ORR was 93.5%, and the CR rate was 61.3%.
A subgroup analysis of CR rates showed the efficacy of this therapy across most prespecified subgroups. However, male patients (n = 14), those with bulky disease of at least 5 cm in diameter (n = 8), and those with double-expressor or double-hit lymphoma (n = 12) had the most inferior CR rates compared with the rest of the population, of 50.0% (95% CI, 23.0%-77.0%), 37.5% (95% CI, 8.5%-75.5%), and 50.0% (95% CI, 21.1%-78.9%), respectively.
The addition of CAR T-cell therapy to the LBCL treatment paradigm has improved the overall prognosis of this population. For example, findings from a 2023 SEER database analysis of 41,219 patients with diffuse LBCL (DLBCL) showed improved overall survival (OS) outcomes in the post-CAR T-cell therapy approval era vs the pre-approval era (HR, 0.951; 95% CI, 0.913-0.990; P = .014).2
“There is a significant difference between those two eras, indicating the benefit brought by the CAR T-cells to [patients with] DLBCL, especially those with relapsed or refractory disease,” presenting author Liang Wang, MD, PhD, contextualized.
Wang is an associate professor in the Department of Hematology at Beijing Tongren Hospital Capital Medical University in China.
However, Wang noted that there is still room for improvement, citing an exploratory long-term survival assessment of the phase 1/2 ZUMA-1 trial (NCT02348216) that showed that axicabtagene ciloleucel (Yescarta) elicited a median lymphoma-related event-free survival (EFS) of 5.7 months (95% CI, 3.1-13.9) and a 5-year EFS rate of 30.3% (95% CI, 21.5%-39.6%).3
CAR2219 is composed of a single-chain variable fragment derived from an anti-CD22 monoclonal antibody fused to a second single-chain variable fragment derived from an anti-CD19 monoclonal antibody.1 Investigators used parallel truncated EGFR expression to detect the expression efficiency of CAR T cells.
“Previous studies have also found that anti-EGFR antibodies may help to eliminate the CAR T cells in case of severe adverse effects [AEs],” Wang added.
Patients in this trial underwent peripheral blood mononuclear cell collection. Bridging therapy prior to CAR2219 infusion was permitted at the choice of the treating physician. Patients then received a 3-day lymphodepletion regimen consisting of cyclophosphamide at 250 mg/m2 per day plus fludarabine at 25 mg/m2 per day, followed by CAR2219 infusion at 2 x 106 cells/kg. Patients who responded to CAR2219 were allowed to receive maintenance therapy with agents such as PD-1 inhibitors 1 month after infusion.
Best ORR at 3 months post-infusion served as the trial’s primary end point. Secondary end points included best CR rate, progression-free survival (PFS), OS, and AEs.
A previously conducted retrospective study evaluated the use of bridging therapy with mitoxantrone liposome plus dexamethasone and polatuzumab vedotin (MPD) with or without rituximab prior to CAR T-cell therapy in patients with LBCL. All patients in that study (n = 10) received 1 cycle of MPD. The median number of prior lines of therapy was 3 (range, 2-5), 7 patients had refractory disease, and the median ECOG performance status before bridging therapy was 2 (range, 1-4). AEs associated with bridging therapy were grade 4 neutropenic fever (n = 2) and COVID-19 infection (n = 1). An efficacy evaluation prior to CAR T-cell infusion showed a disease control rate of 100% (CR, n = 1; PR, n = 5; stable disease [SD], n = 4), relief of lymphopenia-related symptoms, and a median ECOG performance status improvement to 1 (range, 1-2). All patients who received bridging therapy went on to receive subsequent CAR T-cell therapy infusion.
“Thus, we think the MPD regimen was an effective and tolerable regimen,” Wang reported.
Patients enrolled in the phase 2 trial had stage I (6.5%; n = 2), II (6.5%; n = 2), III (13%; n = 4) or IV (74%; n = 23) disease. Most patients had tumor bulk less than 5 cm (74%; n = 23), had refractory disease (74%; n= 23), had an International Prognostic Index score of greater than 2 (71%; n = 22), and had received more than 2 prior lines of therapy (55%; n = 17). Only 2 patients did not receive bridging therapy. Among those who did receive bridging therapy, this treatment resulted in CR (3.4%; n = 1), PR (31%; n = 9), SD (62.1%; n = 18), and progressive disease (3.4%; n = 1). Prognostic markers included double-expressor or double-hit lymphoma (39%; n = 12), p53-positive disease (42%; n = 13), disease invasion of more than 2 extranodal sites (55%; n = 17), lactate dehydrogenase levels above the upper limit of normal (61%; n = 19), and lymphoma present in the bone marrow (13%; n = 4).
At the median follow-up, the median PFS and OS were not reached. The estimated 6-month PFS and OS rates were 83% and 87.1%, respectively. At the data cutoff, 93.5% of treated patients were still alive. Among the 2 deaths that were reported, 1 was attributed to lymphoma progression, and the other was attributed to non-lymphoma causes.
In total, 90% of patients had peak CAR T-cell expansion between days 10 and 14 post-infusion. The median peak CAR T-cell count was 2.35 x 108 cells per liter. CAR T cells were detectable after 3 months in most patients. Additionally, interleukin-6 concentration peaked between days 4 and 7 post-infusion; this level also rebounded after the use of tocilizumab.
Wang highlighted the case of a heavily pretreated male with DLBCL who had progressed on prior PD-1–directed therapy and went on to receive CAR2219. This patient had disease progression at 1 month post-infusion but had CAR T-cell expansion in both blood and neck lymphoma tissue.
“Thus, we think the exhausted CAR T cells may contribute to disease progression,” Wang contextualized.
This patient achieved a CR 2 months after reuse of a PD-1 inhibitor.
“Until now, this patient has been lymphoma free for more than 1 and a half years, and we can detect the CAR T cells in his peripheral blood,” he added.
Overall, Wang explained that CAR2219 was well tolerated in most patients, and investigators reported no AE-related deaths. In total, 74% of patients (n = 23) had grade 1/2 cytokine release syndrome (CRS), with no grade 3 or higher CRS reported. The rate of any-grade immune effector cell–associated neurotoxicity syndrome (ICANS) was 6% (n = 2), and 3% of patients (n = 1) had grade 3 ICANS. Other AEs included neutropenia (any-grade, 93%, n = 29; grade ≥ 3, 90%, n = 28), thrombocytopenia (77%, n = 24; 45%, n = 14), and anemia (71%, n = 22; 32%, n = 10).
Wang noted that the patient with grade 3 ICANS was transferred to the intensive care unit, where he recovered after receiving high-dose steroids, plasma exchange, and cerebrospinal fluid exchange.
“The role of post-infusion maintenance therapy, especially using PD-1 inhibitors or other small molecule agents, needs to be further investigated,” Wang concluded.
Disclosures: Wang had no financial relationships to disclose. He reported that the present trial was supported by grants from the National Natural Science Foundation of China and the National Science and Technology Major Project of China.

Despite policy headwinds earlier in the year, energy storage additions in China and the US are set to continue growing this decade.
The removal of storage mandates in China for renewables and the absence of offsetting drivers were big concerns. However, a new energy storage target was set in September, underlining the commitment of the world’s second largest economy to the sector. China also aims to accelerate the shift from mandates to market-driven growth through the spot market launch and the provincial compensation scheme.
In the US, federal policy shifts brought uncertainty due to frequent changes in import tariffs and new restrictions on the use of equipment from China. Still, market players are quickly adapting to the new environment, supported by domestic battery manufacturing initiatives by leading Korean firms. With slower-than-expected battery demand for electric vehicles, battery makers are shifting focus to stationary energy storage.

Sai, visionary creator Peach Momoko’s popular version of Psylocke from her acclaimed DEMON DAYS SAGA, makes her long-awaited return this January in SAI: DIMENSIONAL RIVALS! The five-issue limited comic book series will be written and drawn by…

In a new study presented at the

Although Apple said in a recent court filing that Jon Prosser “still has not” replied to the company’s July lawsuit against him for allegedly stealing trade secrets, Prosser tells The Verge that he is in communication with Apple about the…
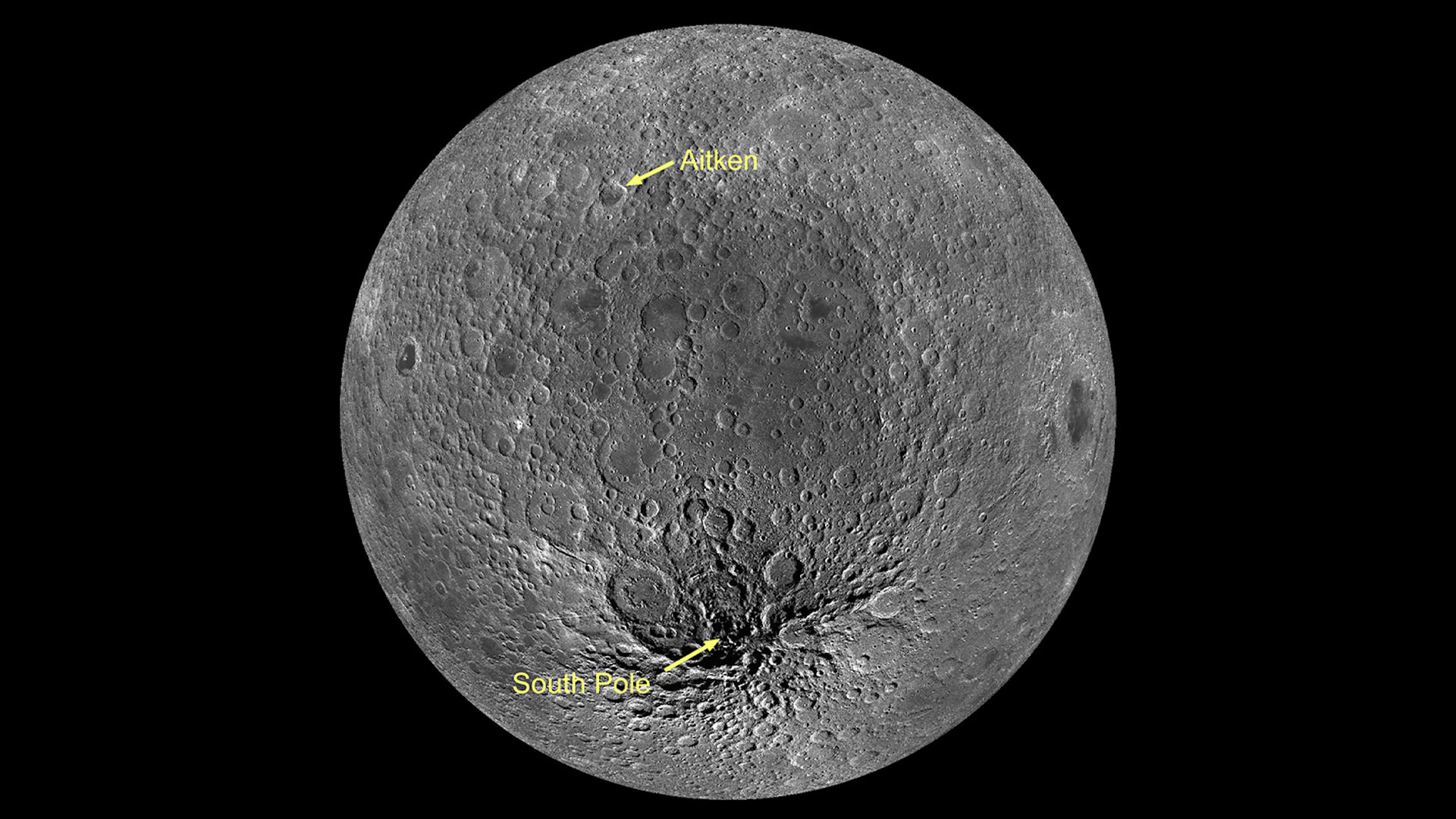
Researchers are pinpointing a moment in the Moon’s history that can help them understand developments on Earth billions of years ago.
University of Arizona lunar scientist Jeff Andrews-Hanna is studying a basin formed by an asteroid’s impact…

Laura Wolvaardt’s brilliant 90 set up a dominant 150-run (DLS method) win for South Africa against Pakistan at the ICC Women’s Cricket World Cup 2025 at the R Premadasa Stadium in Colombo on Tuesday.
The Proteas blasted their way to a…