Translation: L’IATA lance une campagne pour aider les voyageurs à voler en toute sécurité avec des batteries au lithium (pdf)
Xiamen – The International Air Transport Association (IATA) has launched ‘Travel Smart with Lithium Batteries’, a global safety campaign that gives travelers seven simple rules for carrying mobile phones, laptops, power banks, and other lithium-powered devices safely when they fly. The campaign will run on IATA’s website and social channels and is available as white-label assets for airlines, airports, and other partners across the travel ecosystem.
“Lithium-powered devices are safe when handled properly, but they can pose a risk if damaged or packed incorrectly. As more travelers fly with these devices, our Travel Smart with Lithium Batteries campaign will help airlines educate their passengers on the simple rules they must keep in mind when traveling with the electronic devices that have become an essential part of their daily lives,” said Nick Careen, IATA’s Senior Vice President, Operations, Safety and Security.
Travelers Are Carrying More Devices but with Incomplete Knowledge
A recent IATA passenger survey found that most travelers fly with lithium-powered devices:
- 83% of travelers carry a phone
- 60% carry a laptop
- 44% carry a power bank
While 93% of travelers consider themselves knowledgeable on the rules for carrying lithium-powered devices (including 57% rating themselves as very familiar with the rules), critical misconceptions persist:
- 50% incorrectly believe it’s OK to pack small lithium-powered devices in checked luggage
- 45% incorrectly believe it’s OK to pack power banks in checked luggage
- 33% incorrectly believe that there are no power limits on power banks or spare batteries
Seven Simple Safety Rules
The campaign assets highlight seven simple rules every traveler should follow:
- Pack light: Only bring the devices and batteries you really need.
- Stay alert: If a device is hot, smoking, or damaged, tell the crew (or airport staff) immediately.
- Keep devices with you: Always carry phones, laptops, cameras, vapes (if allowed) and other battery-powered items in your hand baggage, not in checked baggage.
- Protect loose batteries: Keep spare batteries and power banks in their original packaging, or cover the terminals with tape to prevent short-circuits.
- Gate check reminder: If your hand baggage is taken at the gate to go in the aircraft baggage hold, remove all lithium batteries and devices first.
- Check battery size: For larger batteries (over 100 watt-hours, such as those used in larger cameras, drones, or power tools), check with your airline as approval may be required.
- Check airline rules: Always confirm your airline’s policies, as requirements may differ in compliance with local regulations.
Industry-Wide Rollout
The multilingual campaign will be rolled out through digital assets that airlines and other partners can adapt and share with passengers, ensuring consistent safety messaging across the industry. A short, animated video, designed to make the rules simple, engaging, and easy to remember, can be used by airlines and airports on their digital and social channels.
Campaign assets will also be available to media and other entities in the aviation value chain to help educate travelers on flying safely with their lithium-powered devices.
> Learn more and download the assets
For more information, please contact:
Corporate Communications
Tel: +41 22 770 2967
Email: corpcomms@iata.org
Notes for Editors:
- IATA (International Air Transport Association) represents some 350 airlines comprising over 80% of global air traffic.
- You can follow us on X for announcements, policy positions, and other useful industry information.
- Fly Net Zero
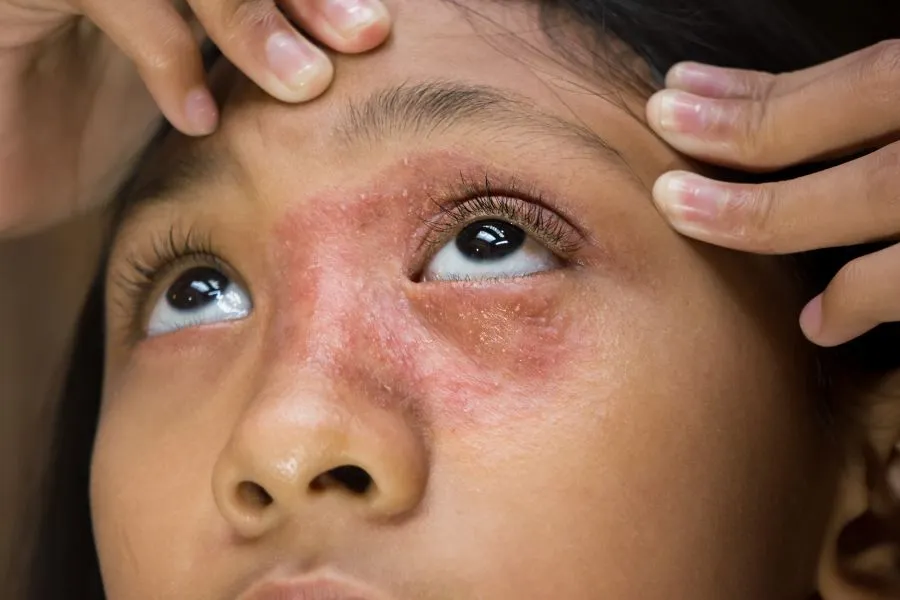
- Common devices that use lithium batteries
Many travelers don’t realize just how many everyday devices contain lithium batteries. Beyond mobile phones and laptops, lithium batteries power a wide range of personal and travel items including tablets, e-readers, wireless headphones, smartwatches, fitness trackers, cameras, portable speakers, power banks, handheld gaming consoles, and electronic styluses. They are also found in everyday personal-care items like electric toothbrushes, shavers, and hair-straighteners, as well as in e-cigarettes, handheld fans, torches, medical devices such as hearing aids and glucose monitors, and compact tools or gadgets like screwdrivers and laser pointers.







00263-8/asset/c1404d50-9e80-463d-9688-a16b7f125c81/main.assets/gr3.jpg)