At the ESMO Congress 2025 in Berlin, Dr. Rocio Garcia-Carbonero (Madrid, Spain) presented the findings of the VIRAGE trial (2216MO) — a randomized phase IIb, open-label study evaluating the oncolytic adenovirus VCN-01 combined with standard-of-care gemcitabine and nab-paclitaxel (GA) in patients with metastatic pancreatic ductal adenocarcinoma (mPDAC). The study marks a pivotal advance in the application of oncolytic virotherapy for a disease long characterized by therapeutic resistance and dismal outcomes.
Background
VCN-01 (zabilugene almadenorepvec) is an engineered oncolytic adenovirus designed to selectively replicate within tumor cells and express hyaluronidase, an enzyme that degrades hyaluronic acid—a key component of the dense pancreatic tumor stroma. By dismantling this fibrotic barrier, VCN-01 aims to enhance chemotherapy delivery, immune cell infiltration, and antitumor immune responses, addressing the stromal desmoplasia that limits efficacy in pancreatic cancer.
Following promising safety and early efficacy signals in prior studies, VIRAGE was designed to test whether incorporating two intravenous doses of VCN-01 into a GA backbone could prolong survival in chemotherapy-naïve patients with metastatic disease.
Methods
A total of 112 patients with previously untreated mPDAC were randomized 1:1 into two treatment arms:
- Arm I (Control): Standard GA administered on days 1, 8, and 15 of each 28-day cycle.
- Arm II (Experimental): Two IV doses of VCN-01 (1×10¹³ viral particles each), given one week before cycles 1 and 4 of GA.
Primary endpoints were overall survival (OS) and safety. Secondary endpoints included progression-free survival (PFS), objective response rate (ORR), duration of response (DoR), and Ca19.9 biomarker kinetics.
Two efficacy populations were analyzed
- mITT (modified intent-to-treat): ≥1 dose of GA (Arm I) or ≥1 dose of VCN-01 (Arm II)
- FAS (full analysis set): ≥1 dose of GA (Arm I) or ≥1 dose of both VCN-01 and GA (Arm II)
The trial had 80% power to detect OS differences with a two-sided α of 0.1.
Results
In the mITT population, patients treated with VCN-01 + GA achieved a median OS of 10.6 months versus 8.6 monthswith GA alone (HR 0.69; 95% CI 0.42–1.12; P = 0.196). In the FAS cohort, the survival benefit became more evident: 10.8 vs 8.6 months (HR 0.57; 95% CI 0.34–0.96; P = 0.055).
Median PFS improved from 4.6 to 7.0 months (HR 0.55; 95% CI 0.34–0.88; P = 0.011), while DoR more than doubled (5.4 vs 11.2 months; P = 0.004). The ORR increased from 31.3% to 39.6%, indicating not only more responses but also their longer durability.
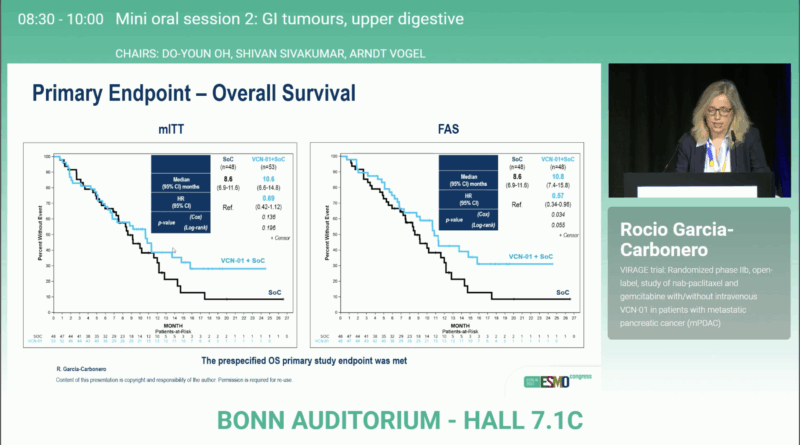
Among patients who reached cycle 4, outcomes were even more favorable:
- OS: 14.8 vs 11.6 months (HR 0.44; 95% CI 0.21–0.92; P = 0.046)
- PFS: 11.2 vs 7.4 months (HR 0.48; 95% CI 0.25–0.91; P = 0.017)
These data suggest a dose-dependent therapeutic effect and confirm the biological activity of the second viral infusion.
Safety
Treatment with VCN-01 was well tolerated. The most common virus-related adverse events were flu-like symptoms (13.2%), elevated transaminases (5.7%), and drug-induced liver injury (3.8%). All VCN-01-related serious adverse events (n=13) were transient and resolved with standard care.
Importantly, viral genome analysis verified successful replication and bioactivity of both VCN-01 doses, a crucial mechanistic validation of the therapeutic concept. Hematologic and non-hematologic toxicities were consistent with the known profile of gemcitabine/nab-paclitaxel, with no unexpected safety signals.
Interpretation
The VIRAGE trial achieved its primary endpoints, demonstrating that the addition of VCN-01 to standard chemotherapy improves overall survival, progression-free survival, and duration of response in metastatic pancreatic cancer. The benefit observed among patients completing both viral doses highlights the importance of sustained viral activity and microenvironmental remodeling in achieving clinical efficacy.
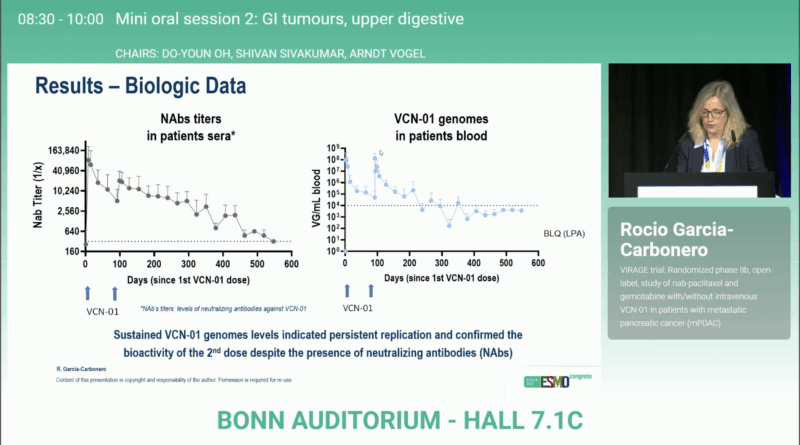
By enzymatically disrupting the dense extracellular matrix, VCN-01 enhances drug perfusion and immune activation, addressing one of the most critical biological barriers in PDAC treatment. This supports the broader strategy of combining stroma-modifying virotherapy with cytotoxic or immune-based regimens.
You can read the full abstract here.
Conclusion
The results of the VIRAGE trial underscore a promising new frontier for patients with metastatic pancreatic cancer. The combination of VCN-01 with gemcitabine and nab-paclitaxel delivered not only meaningful survival gains but also confirmed the mechanistic synergy between oncolytic virotherapy and chemotherapy.
For a malignancy with limited progress over the past decade, these data represent a proof of concept that microenvironment modulation through viral engineering can translate into tangible clinical benefit.
Future phase III studies and potential combinations with immune checkpoint inhibitors will further define the role of VCN-01-based strategies in reshaping the treatment landscape of pancreatic cancer.
Read more about BREAKWATER Study Results at ESMO 2025: ctDNA Clearance and Resistance Evolution in BRAF V600E–Mutant Colorectal Cancer on OncoDaily.