A Dutch study presented at the 20th European AIDS Conference (EACS 2025) in Paris compared the health-related quality of life (HRQoL) in around 500 older people with HIV with the same number of carefully matched people without HIV for the last…
Blog
-
Durvalumab Plus FLOT Ups Survival in Early Upper-GI Cancer – Medscape
- Durvalumab Plus FLOT Ups Survival in Early Upper-GI Cancer Medscape
- AstraZeneca – IMFINZI-Based Regimen Reduced The Risk Of Death By 22% In Early Gastric Cancer Versus. Chemotherapy Alone In MATTERHORN Phase III Trial TradingView
- MATTERHORN Trial at ESMO 2025: Durvalumab Plus FLOT in Resectable Gastric and GEJ Adenocarcinoma Oncodaily
- Imfinzi Plus Chemo Improves Survival in G/GEJ Adenocarcinoma CUREtoday.com
- Durvalumab Plus Chemotherapy Sets New Standard in Resectable Gastric and GEJ Cancer Pharmacy Times
Continue Reading
-

What was Steve Bridges’ cause of death? TikTok comedian dead at 41
TikToker Steve Bridges has passed away at age 41.
His wife, Chelsey Bridges, shared the news on his Instagram page on Friday, Oct. 17.
“A light gone too soon. I love you Steve,” she captioned her video, in which she revealed that the Illinois…
Continue Reading
-
Phase 2 BNT111/Cemiplimab Data Prove Positive in PD-(L)1-Relapsed/Refractory Melanoma
The addition of BNT111 to cemiplimab-rwlc (Libtayo) led to an objective response rate (ORR) of 18.1% (95% CI, 10.9%-27.4%; P = .0115) in patients with PD-(L)1-relapsed/refractory melanoma, allowing investigators to reject the null hypothesis of an ORR below 10%, according to data from the phase 2 BNT111-01 trial (NCT04526899) that were presented at the
2025 ESMO Congress .1Best responses included a complete response (CR) rate of 11.7%, partial response (PR) rate of 6.4%, and stable disease (SD) rate of 37.2%. The disease control rate (DCR) was 55.3% (95% CI, 44.7%-65.6%).
With median follow-up of 15.7 months (range, 0.2-42.2), the median progression-free survival (PFS) was 3.1 months (95% CI, 1.7-6.9); the 24-month PFS rate was 24.9% (95% CI, 14.9%-36.1%). The median overall survival (OS) was 20.7 months (95% CI, 14.4-28.3); the 24-month OS rate was 47.8% (95% CI, 36.4%-58.4%).
“The results indicated statistically significant improvement of BNT111 plus cemiplimab vs an assumed historical control ORR of 10% in heavily pretreated, PD-(L)1-relapsed/refractory advanced or metastatic cutaneous non-acral melanoma,” lead study author Paolo Ascierto, MD, full professor of oncology at the University of Napoli Federico II, and director of the Department of Melanoma, Cancer Immunotherapy and Development Therapeutics at the Istituto Nazionale Tumori IRCCS Fondazione Pascale in Naples, Italy, said in a presentation.
What Is the Development History of BNT111 in Melanoma?
The company announced
the positive topline results back in July 2024, 3 years after BNT111 received fast track designation from the FDA for the potential treatment of patients with advanced melanoma.2,3BNT111 is an investigational uridine RNA-based lipoplex cancer immunotherapy targeting the nonmutated, tumor-associated antigens NY-ESO-1, MAGE-A3, Tyrosinase, and TPTE.1
BNT111-01 was designed as an open-label, randomized, multi-center, interventional trial to evaluate the activity and safety of BNT111 plus cemiplimab as second-line therapy in patients with unresectable stage III or IV melanoma who had progressed on prior PD-(L)1 therapy.
To be eligible for the trial patients had to have measurable disease, serum lactase dehydrogenase levels below the upper limit of normal, and received up to 5 prior lines of therapy including ipilimumab (Yervoy). Notably, patients had to enter the trial within 6 months of confirmed disease progression and have received at least 12 weeks of treatment, which must have included a BRAF-based combination for patients with BRAF-mutant disease.
A total of 180 patients were randomly assigned 2:1:1 to treatment with BNT111 plus cemiplimab (n = 94; arm 1), BNT111 monotherapy (n = 46; arm 2), or cemiplimab monotherapy (n = 44; arm 3), all for up to 24 months. Patients in the monotherapy arms were allowed to add the other agent upon confirmation of disease progression.
The primary end point was ORR by blinded independent central review per RECIST 1.1 in arm 1, which was compared with an assumed historic control ORR of 10%. Secondary end points were ORR in arms 2 and 3, duration of response, DCR, time to response, PFS, OS, safety, tolerability, and patient-reported outcomes.
Patients were followed for safety for 90 days and OS every 3 months for up to 48 months from the last randomization.
What Were the Baseline Characteristics of the Trial Population?
Baseline characteristics of the combination arm revealed that the median age was 64.0 (range, 18-84) and most patients were male (63.8%) and had an ECOG performance status of 0 (78.7%). The majority also had stage IV disease at baseline (97.9%), M1c disease (44.7%), and between 2 and 5 prior therapies (56.4%). Patients also were PD-(L)1 refractory (56.4%), and had liver metastases (25.5%), BRAF V600 mutations (28.7%), prior BRAF/MEK therapy (18.1%), and prior ipilimumab (48.9%).
“All patients were PD-(L)1 relapsed/refractory and had received one or multiple prior therapies with half of patients being CTLA4 pretreated,” Ascierto said.
How Did the Regimen Compare With Each Agent Alone?
“BNT111 also indicated clinical activity as monotherapy.” The ORR was 17.4% (95% CI, 7.8%-31.4%) with BNT111 monotherapy (n = 46), which included best responses of CR (13.0%), PR (4.3%), and SD (41.3%). The DCR was 58.7% (95% CI, 43.2%-73.0%). With cemiplimab monotherapy (n = 44), the ORR was 13.6% (95% CI, 5.2%-27.4%), with best responses of CR (4.5%), PR (9.1%), and SD (34.1%). The DCR was 47.7% (95% CI, 32.5%-63.3%).
Median follow-up was 11.8 months (range, 0.6-38.4) in the BNT111 arm and 16.9 months (range, 1.9-39.5) in the cemiplimab arm. In the BNT111 monotherapy arm, the median PFS was 2.8 months (95% CI, 2.6-4.7); the 24-month PFS rate was 20.9% (95% CI, 8.0%-37.9%). The median OS was 13.7 months (95% CI, 10.2-24.6); the 24-month OS rate was 37.6% (95% CI, 22.7%-52.5%). In the cemiplimab monotherapy arm, the median PFS was 3.2 months (95% CI, 1.5-5.0); the 24-month PFS rate was 10.6% (95% CI, 1.1%-32.5%). The median OS was 22.3 months (95% CI, 14.6-33.2); the 24-month OS rate was 43.1% (95% CI, 26.5%-58.7%).
What Was the Safety Profile of the Combination?
With respect to safety in the combination arm (n = 92) treatment-emergent adverse effects (TEAEs) associated with cytokine release included pyrexia (any grade, 76.1%; grade ≥3, 0%), hypertension (any grade, 12.0%; grade ≥3, 6.5%), hypotension (any grade, 13.0%; grade ≥3, 2.2%), fatigue (any grade, 25.0%; grade ≥3, 3.3%), cytokine release syndrome (any grade, 8.7%; grade ≥3, 1.1%), and chills (any grade, 51.1%; grade ≥3, 0%).
TEAEs leading to dose reduction (9.8%), interruption (29.3%), and discontinuation (6.5%) also occurred. TEAEs occurred in 98.9% of cases and were deemed related to BNT111 in 97.8% (grade ≥3, 18.5%) and cemiplimab in 71.7% (grade ≥3, 14.1%).
“BNT111, both as a monotherapy and in combination therapy, exhibited a manageable safety profile, which is primarily driven by the induction of cytokines through toll-like receptors by the single-stranded RNA immunotherapy,” Ascierto said in conclusion.
Disclosures: Ascierto disclosed serving as a consultant or advisory role for Bristol-Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, Merck Serono, Pierre-Fabre, Sun Pharma, Immunocore, Italfarmaco, Boehringer-Ingelheim, Regenerson, Pfizer, Nouscom, Lunaphore, Medicenna, Bio-Al Health, ValoTx, Replimune, Bayer, Erasca, Philogen, BioNTech, Anaveon, Genmab, Menarini, Incyte, and ImCheck Therapeutics; research funding from Bristol-Myers Squibb, Roche-Genentech, Pfizer, Regeneron, and Medicenna; travel support from Pfizer, Bio-Al Health, Replimune, MSD, Pierre Fabre, and Philogen; and non-financial interests as president of the Fondazione Melanoma Onlus, president of the Campania Society of ImmunoTherapy of Cancer, member of the steering committee of the Society of Melanoma Research, and a member of the Board of Directors for the Society of Immuno-Therapy of Cancer.
References
- Ascierto PA, Grabbe S, Guida M, et al. Primary results from a randomized phase II trial of BNT111 in combination with cemiplimab with calibrator monotherapy arms in anti-PD-(L)1 relapsed/refractory melanoma. Presented at: 2025 ESMO Congress; October 17-21, 2025; Berlin, Germany. Abstract 1605MO.
- BioNTech announces positive topline phase 2 results for mRNA immunotherapy candidate BNT111 in patients with advanced melanoma. News release. BioNTech SE. July 30, 2024. Accessed October 18, 2025. https://investors.biontech.de/news-releases/news-release-details/biontech-announces-positive-topline-phase-2-results-mrna
- BioNTech receives FDA fast track designation for its FixVac candidate BNT111 in advanced melanoma. News release. BioNTech SE. November 19, 2021. Accessed October 18, 2025. https://investors.biontech.de/news-releases/news-release-details/biontech-receives-fda-fast-track-designation-its-fixvac
Continue Reading
-
New Zealand vs Pakistan Women’s Cricket World Cup 2025 match abandoned
Rain scuppered any hope of a result in Colombo as New Zealand’s clash with Pakistan on Saturday was abandoned – a result that guarantees South Africa’s place in the Women’s Cricket World Cup 2025 semi-finals.
With eight points to their…
Continue Reading
-

Fondation Cartier Reopens in Paris with Bold Art
In the most highly anticipated opening of the season, the Fondation Cartier for Contemporary Art will unveil its new Parisian address – and it’s not…
Continue Reading
-

T-DXd vs T-DM1 in HER2+ Early BC
DESTINY-Breast05 (NCT04622319), presented by Dr. Charles E. Geyer (Pittsburgh, United States of America) at the ESMO Congress 2025, is a pivotal phase 3, open-label, randomized trial evaluating trastuzumab deruxtecan (T-DXd) versus the standard-of-care trastuzumab emtansine (T-DM1) in patients with HER2-positive early breast cancer (eBC) who had residual invasive disease after neoadjuvant therapy. The study was designed to determine whether T-DXd could improve long-term outcomes for this high-risk population compared with T-DM1, the established post-neoadjuvant standard of care
Background
Patients with HER2-positive early breast cancer who have residual invasive disease following neoadjuvant chemotherapy and anti-HER2 therapy face a high risk of recurrence, particularly distant relapse. T-DM1 became the standard post-neoadjuvant treatment following the KATHERINE trial, but outcomes remain suboptimal for patients with high residual disease burden. Trastuzumab deruxtecan (T-DXd), a next-generation HER2-directed antibody–drug conjugate with a potent topoisomerase I inhibitor payload, has shown marked efficacy in metastatic settings, prompting investigation into its use in the early disease setting to reduce recurrence risk.
Methods
In DESTINY-Breast05, 1,635 patients with HER2-positive eBC and residual invasive disease after neoadjuvant taxane-based chemotherapy and HER2-targeted therapy were randomized 1:1 to receive either:
- T-DXd (5.4 mg/kg) every 3 weeks,for a total of 14 cycles.
- T-DM1 (3.6 mg/kg) every 3 weeks,for a total of 14 cycles.
Eligible patients were considered high risk for recurrence, defined by clinical stages T4, N0–3, M0 or T1–3, N2–3, M0 at presentation, or residual nodal disease after neoadjuvant therapy.
The primary endpoint was invasive disease-free survival (IDFS), with disease-free survival (DFS) as a key secondary endpoint. Additional endpoints included overall survival (OS), distant recurrence-free interval, brain metastasis–free interval (BMFI), and safety.
Results
At the data cutoff of July 2, 2025, median follow-up was 29.9 months in the T-DXd arm and 29.7 months in the T-DM1 arm.
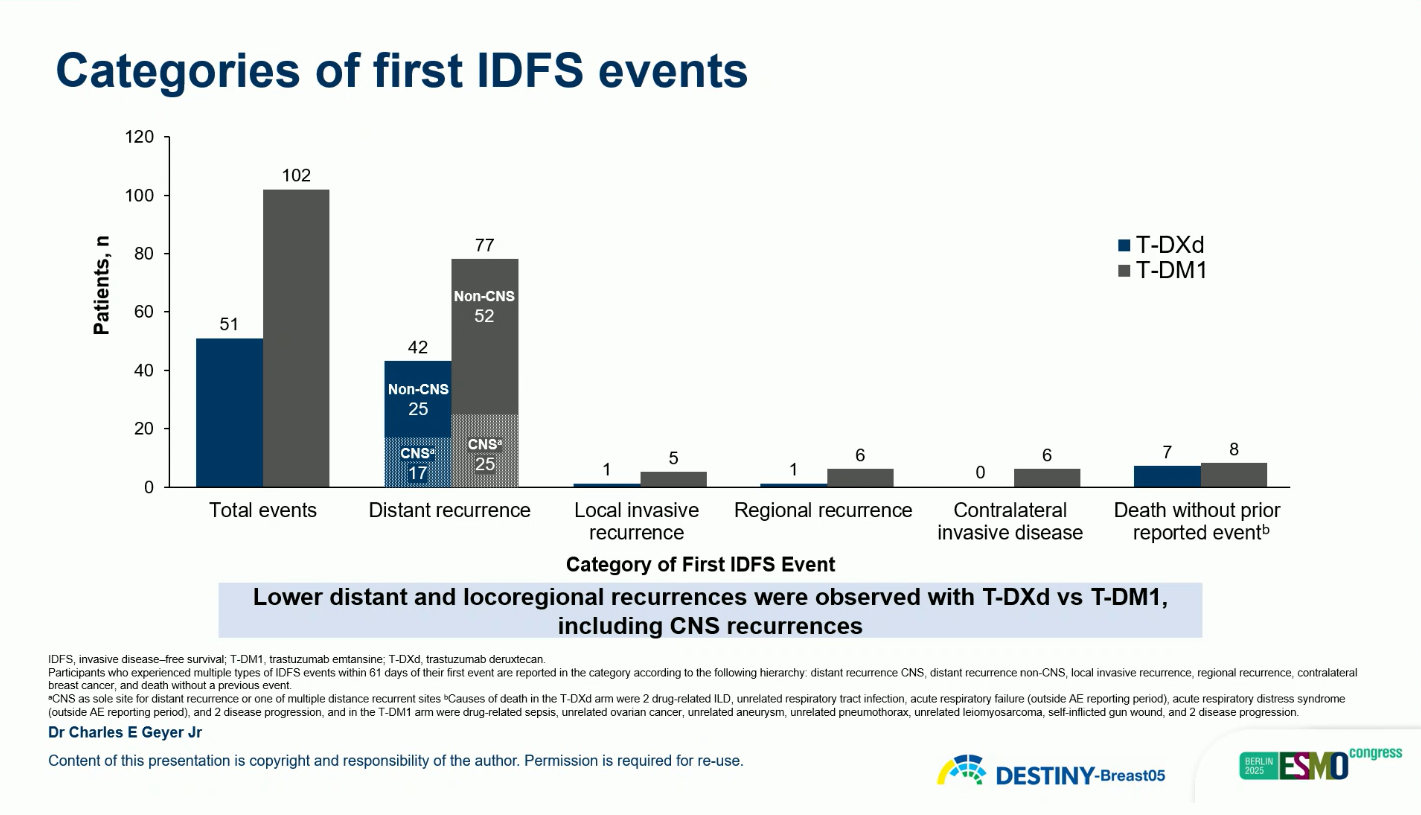
- IDFS events: 6.2% with T-DXd vs 12.5% with T-DM1
- DFS events: 6.4% vs 12.6%
- Hazard ratio (HR): 0.47 for both IDFS and DFS (95% CI: 0.34–0.66; p < 0.0001)
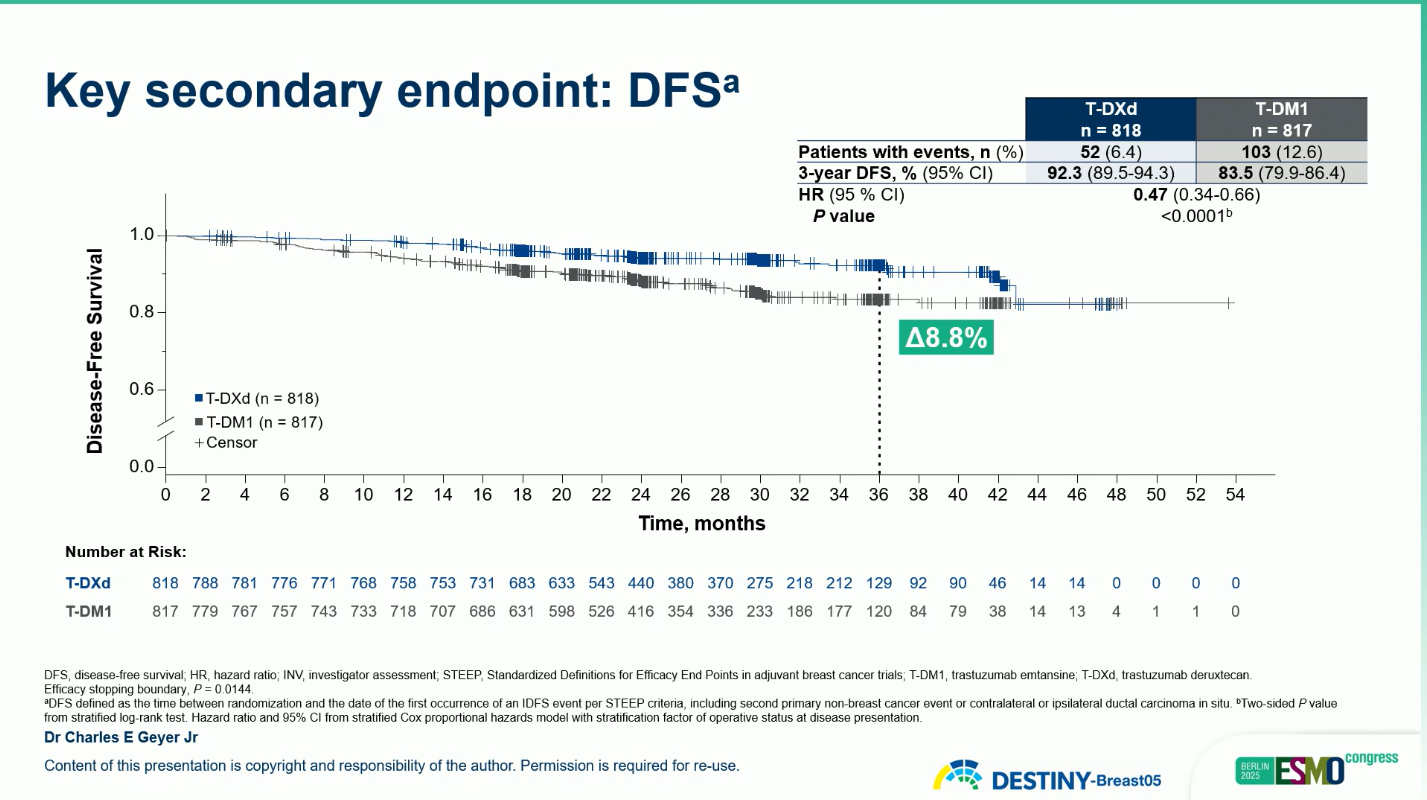
Similarly, the key secondary endpoint of disease-free survival (DFS) demonstrated a parallel benefit, with a hazard ratio of 0.47 (95% CI, 0.34–0.66; p < 0.0001). The 3-year DFS rate was 92.3% with T-DXd compared with 83.5% with T-DM1, corresponding to an 8.8% absolute gain.
These findings represent a 53% reduction in risk of invasive disease recurrence or death with T-DXd compared with T-DM1.
A clinically meaningful improvement in BMFI was also observed (HR 0.64; 95% CI 0.35–1.17), suggesting enhanced control of central nervous system relapse.
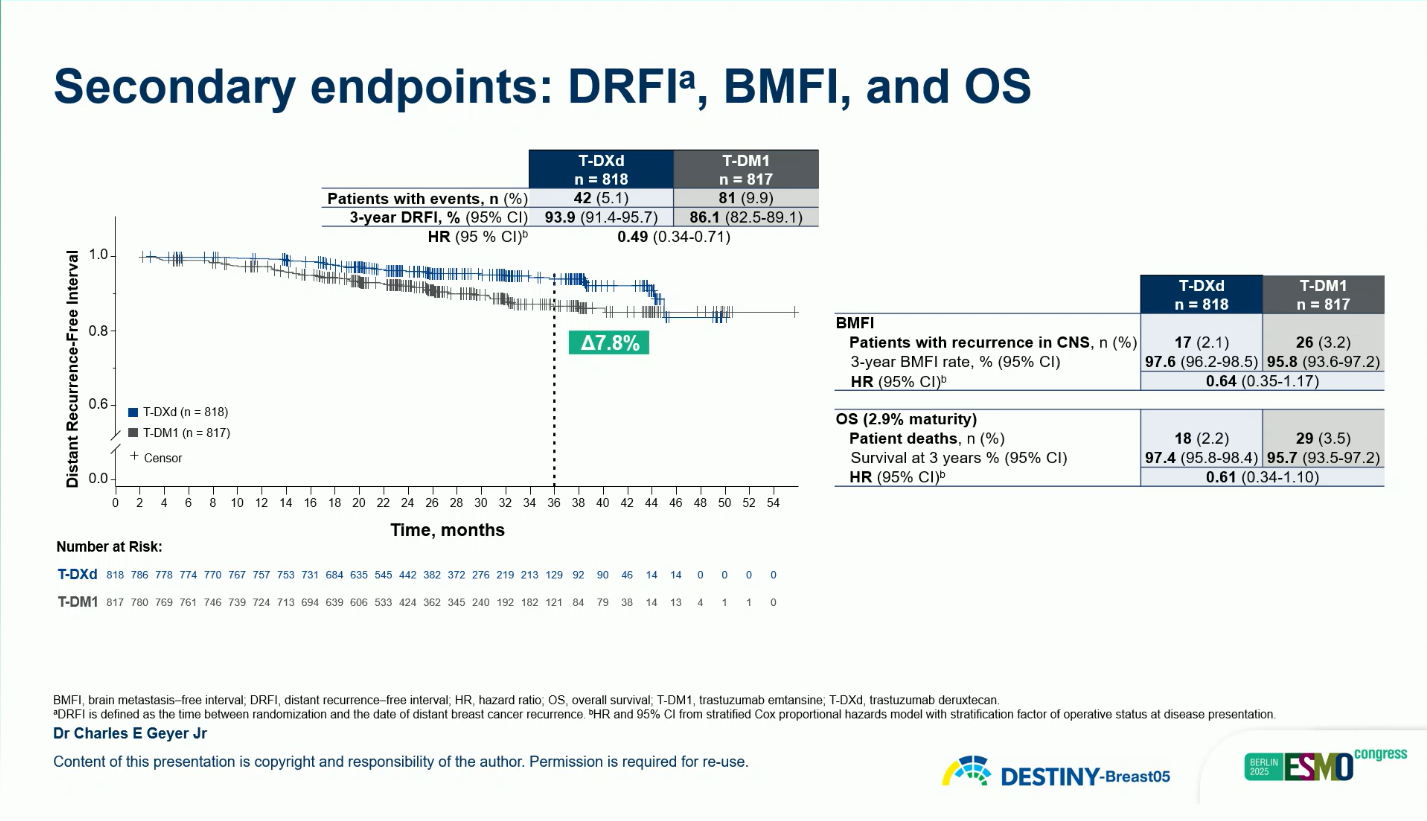
Safety
The overall safety profile of T-DXd was manageable and consistent with prior studies.
- Grade ≥3 TEAEs: 50.6% (T-DXd) vs 51.9% (T-DM1)
- Adjudicated interstitial lung disease (ILD): 9.6% (2 grade 5 cases) vs 1.6% (none grade 5)
- Treatment-related deaths: 0.4% (T-DXd) vs 0.6% (T-DM1)
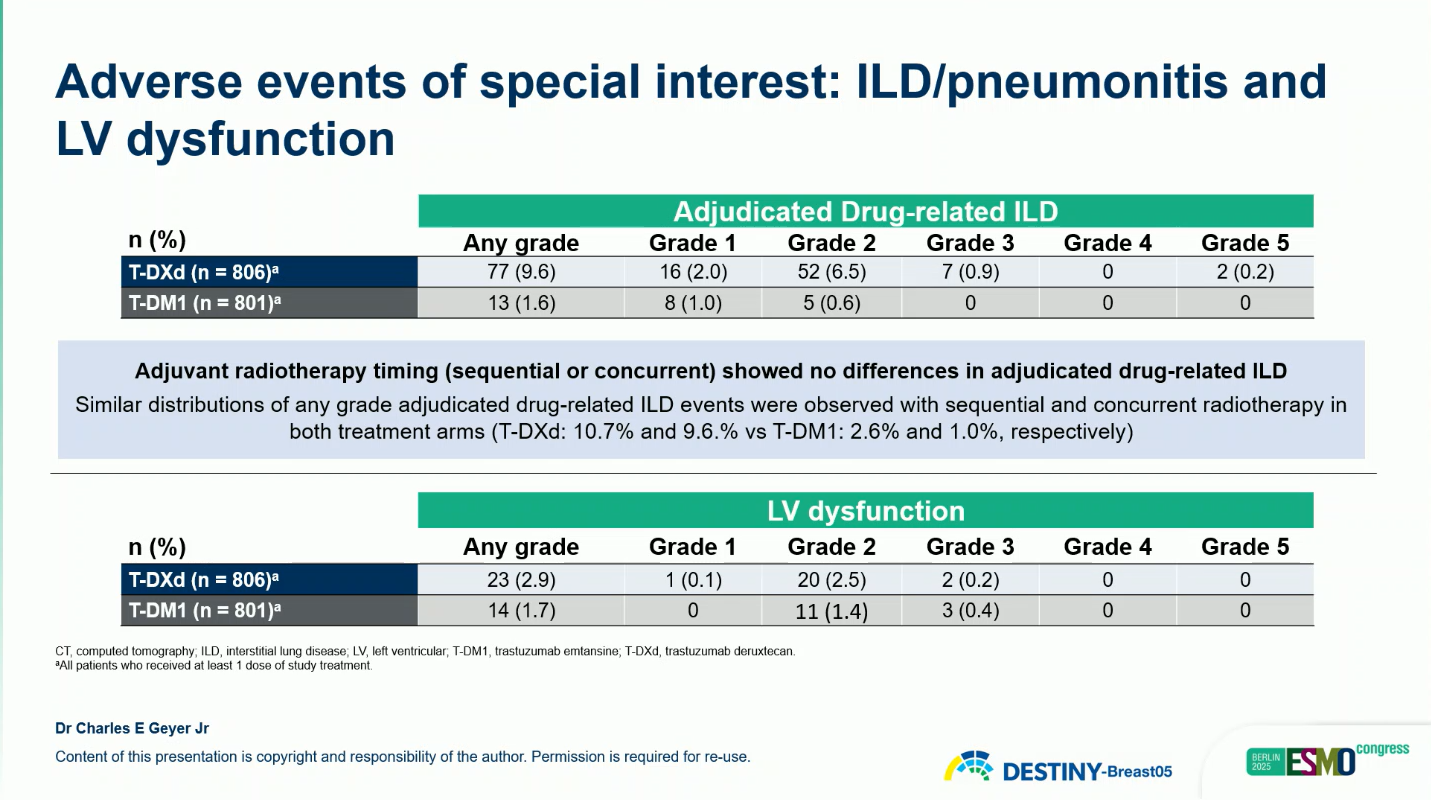
Most ILD events were grade 1–2 and resolved with treatment modification or corticosteroids. No new safety signals were identified.
Conclusions
The DESTINY-Breast05 trial demonstrated that trastuzumab deruxtecan (T-DXd) offers a statistically significant and clinically meaningful improvement in both invasive disease-free survival (IDFS) and disease-free survival (DFS) compared with trastuzumab emtansine (T-DM1) in patients with HER2-positive early breast cancer who had residual invasive disease following neoadjuvant therapy.
These findings mark a pivotal advance in the post-neoadjuvant management of HER2-positive breast cancer. By extending the proven efficacy of T-DXd beyond the metastatic setting into early-stage, high-risk disease, the results highlight its potential to redefine the standard of care for patients who previously had limited options after incomplete response to neoadjuvant therapy. Importantly, the benefit was consistent across all major subgroups, including hormone receptor–positive and –negative disease, as well as across regions and baseline disease characteristics, underscoring the robustness of the findings.
You can read the full abstract here.
Continue Reading
-

8 songs from the 60s and 70s that instantly transport Boomers back to better times – VegOut
Watch a room of 70-somethings when “Mrs. Robinson” starts. Shoulders straighten, eyes soften, lips move to words unvisited for decades. For three minutes and thirty seconds, mortgages and medications vanish. They’re nineteen again,…
Continue Reading
-

Decart Brings Real-Time AI To Real-Time Creators At TwitchCon
San Diego, CA. October 17, 2025. Posing for the AI Camera at Twitch Con.
Charlie Fink
At TwitchCon San Diego, the Israeli startup Decart staged a live demonstration of something I’ve not seen before: real-time generative AI video. Partnering…
Continue Reading
-

ITM-11 Meets Primary and Secondary End Points in Final Trial Data in Patients with GEP-NETs | Targeted Oncology
Final analysis from the
phase 3 COMPETE trial (NCT03049189)1revealed that 177-Lu-edotretide (ITM-11) vs everolimus met its primary and secondary endpoints in patients with gastroenteropancreatic neuroendocrine tumors (GEP-NETs).The final analysis was announced at the 2025 European Association of Nuclear Medicine Annual CongressSociety for Medical Oncology (ESMO) Congress on October 18, 2025.21 The results were presented by Jaume Capdevila, MD, PhD, Vall d’Hebron University Hospital.
The primary endpoint was progression-free survival (PFS), which was reached with statistically significant and clinically meaningful improvement. The median PFS was significantly longer in patients who tookadministered ITM-11 compared to those who tookadministered everolimus. The secondary endpoint of the trial was overall survival (OS), which was also identified to be higher in patients who tookwere administered ITM-11 vs everolimus.2
There was a total of 207 patients in the ITM-11 group and 102 patients in the everolimus group. , respectively. The median ages of both groups were 65 (ITM-11), and 61 (everolimus). Majority of patients in both groups were male. The majority of patients had grade 2, non-functional GEP-NETs and had received prior therapy.1,2
What Were the Results of the COMPETE Trial?
COMPETE met its primary endpoint of PFS, which proved to be significantly longer in patients treated with ITM-11 vs everolimus. The central assessment was 23.9 vs 14.1 months (HR, 0.67; 95% CI, 0.48–0.95; P=.022).; HR .67, 95% CI [.48, .95]). The local assessment was 24.1 vs 17.6 months (P=.010; HR, 0.66; 95% CI, 0 [.48–0, .91] P =.010;).2
In the subgroup analysis of PFS by tumor origin, mPFS was found to be numerically longer in GE-NETs and P-NETs in the ITM-11 arm. In GE-NETs the mPFS was 23.9 vs 12 months (P=.090; HR 0.64, 95% CI, 0i [.38–, 1.08;] P=.090;). In P-NETs the mPFS was 24.5 vs 14.7 months (HR, 0.70, P=.114; HR .70, 95% CI, 0 [.45–, 1.09; P=.114;]).2
It was also identified that mPFS was numerically longer in grade 1 and significantly longer in grade 2 tumors in the ITM-11 arm. Grade 1 was 30 vs 23.7 months (P=.753; HR, 0.89, 95% CI, 0. [.42–, 1.8; 7]P =.753;), and grade 2 was 21.7 vs 9.2 months (P=.0003; HR 0.55l , 95% CI, 0i [.37–0, .82] P =.0003).2
In exploring PFS by prior therapy, it was identified that mPFS was numerically longer in the first line and significantly longer in the second line in the ITM-11 arm. First line data showed the mPFS was not reached in the ITM-11NR vs 18.1 months (P=.249; HR, 0.60, 95% CI, 0 [.25–, 1.45; P =.249]), and second line data showed 23.9 vs 14.1 months (P=.039; HR, 0.68; , 95% CI, 0 [.47–0,, 98] P=.039).2
Overall response rates (ORR), one of the secondary endpoints of the trial, was found to be significantly higher in the ITM-11 arm. Central assessment was 21.9% vs 4.2% (P<.0001), and local assessment was 30.5% vs 8.4% (P<.0001).2
What Adverse Events Were Reported?
Adverse events (AEs) related to the drug study were experienced by 82% of patients ITM-11 group, and 97% of patients in the everolimus group. The most common AEs reported were nausea (30% vs 10.1%), diarrhea (14.3% vs 35.4%), asthenia (25.3% vs 31.3%), and fatigue (15.7% vs 15.2%). These AEs were expected based on the known safety profile of ITM-11.2
AEs leading to premature study discontinuation wereas 1.8% vs 15.2% among both groups, respectively, dose modification or discontinuation wereas 3.7% vs 52.5%, among both groups, and the amount of patients with delayed study drug administration due to toxicity was 0.9% in the ITM-11 group, and none 0% in the everolimus group.2
Dosimetry data showed targeted tumor uptake with low exposure to healthy organs, with normal organ absorbed doses well below safety thresholds.
What Were the Patient Criteria?
Patient inclusion criteria included being 18 or older, having well-differentiated, non-functional GE-NET or functional/non-functional P-NET;, grade 1/2 unresectable or metastatic, progressive, SSRT-positive+ disease; and , being treatment-naive to first-line therapies or progressing , or progressed under prior second-line therapies.1,2
Morphologic imagining was conducted in 3-month intervals. The PFS follow-up was done every 3 months after the first 30 days. Long-term follow-up was done every 6 months.
“With these data combining extensive dosimetry information from more than 200 patients included in a prospective trial, ITM is laying the groundwork for improved therapeutic decision-making by providing important insights into tumor uptake and treatment variability,” Emmanuel Deshayes, MD, PhD, professor in biophysics and nuclear medicine at the Montpellier Cancer Institute in France, said in a news release.2 “It may offer clinically meaningful implications for optimizing individualized patient management.”
Dosimetry data from COMPETE shaped the design of ITM’s phase 3 COMPOSE (NCT04919226)4 trial, with ITM-11 in well-differentiated, aggressive grade 2 or grade 3 SSTR-positive+ GEP-NET tumors, as well as the upcoming phase 1 pediatric KinLET (NCT06441331) study in SSTR-positive+ tumors.
DISCLOSURES: Capdevila noted grants and/or research support from Advanced Accelerator Applications, AstraZeneca, Amgen, Bayer, Eisai, Gilead, ITM, Novartis, Pfizer, and Roche; participation as a speaker, consultant, or advisor for Advanced Acclerator Applications, Advanz Pharma, Amgen, Bayer, Eisai, Esteve, Exelixis, Hutchmed, Ipsen, ITM, Lilly, Merck Serono, Novartis, Pfizer, Roche, and Sanofi; position as advisory board member for Amgen, Bayer, Eisai, Esteve, Exelixis, Ipsen, ITM, Lilly, Novartis, and Roche; and a leadership role and chair position for the Spanish Task Force for Neuroendocrine and Endocrine Tumours Group (GETNE).
REFERENCES:
1.Capdevilla J, Amthauer H, Ansquer C, et al. Efficacy, safety and subgroup analysis of 177Lu-edotreotide vs everolimus in patients with grade 1 or grade 2 GEP-NETs: Phase 3 COMPETE trial. Presented at: 2025 ESMO Congress; October 17-20, 2025; Berlin, Germany. Abstract 1706O
2. ITM presents dosimetry data from phase 3 COMPETE trial supporting favorable efficacy and safety profile with n.c.a. 177Lu-edotreotide (ITM-11) in patients with gastroenteropancreatic neuroendocrine tumors at EANM 2025 Annual Congress. News release. ITM. October 8, 2025. Accessed October 18, 2025.
https://tinyurl.com/3nuscs4m 3. Lutetium 177Lu-Edotreotide versus best standard of care in well-differentiated aggressive grade-2 and grade-3 gastroenteropancreatic neuroendocrine tumors (GEP-NETs) – COMPOSE (COMPOSE). ClinicalTrials.gov. Updated September 10, 2025. Accessed October 18, 2025.
https://www.clinicaltrials.gov/study/NCT04919226 4. Phase I trial to determine the dose and evaluate the PK and safety of lutetium Lu 177 edotreotide therapy in pediatric participants with SSTR-positive tumors (KinLET). ClinicalTrials.gov. Updated September 19, 2025. Accessed October 18, 2025.
https://www.clinicaltrials.gov/study/NCT06441331 Continue Reading
