…


Findings presented at the International VT Symposium and published simultaneously in Circulation.
CARDIFF-BY-THE-SEA, Calif. and PHILADELPHIA, Oct. 10, 2025 /PRNewswire/ — Field Medical, Inc. today announced that Circulation has published six-month outcomes from the Ventricular Catheter Ablation Study (VCAS), the first-in-human evaluation of its FieldForce™ Ablation System for ventricular tachycardia (VT). Results were also presented as a late-breaking trial at the 20th Annual International Symposium on Ventricular Arrhythmias (VT Symposium).
The FieldForce™ Ablation System by Field Medical: This next-generation PFA system is designed for transmural lesion creation, offering a novel approach to VT ablation.
Results at Six Months
VCAS is a prospective, multicenter feasibility trial evaluating the safety and performance of the FieldForce Ablation System in patients with VT. Unlike conventional approaches, the system delivers a proprietary high-voltage, short-pulse waveform designed to penetrate dense scar tissue while minimizing thermal injury.
Key Findings:
“While this remains an initial feasibility study, the six-month outcomes are highly encouraging. Achieving 82% freedom from recurrence and a 98% reduction in arrhythmia burden with a nonthermal, tissue-selective energy is a meaningful result in VT therapy. Importantly, this is the first time we’ve seen evidence that PFA can reach deep, transmural scar tissue in the ventricle, a long-standing challenge with existing energy sources,” said Vivek Reddy, M.D., co-principal investigator, lead author, and electrophysiologist at Mount Sinai, New York. “These findings give me cautious optimism that with continued refinement, this approach could represent an important advance in the treatment of scar-related VT.”
With U.S. Food and Drug Administration’s (FDA) Breakthrough Device designation and acceptance into the FDA Total Product Life Cycle (TAP) Pilot Program, Field Medical is advancing this program toward a pivotal trial and a rigorous evaluation of high-voltage focal PFA in VT.
“It is rare for initial feasibility data to be published in Circulation, and this underscores both the rigor and the significance of the work,” said Steven Mickelsen, M.D., founder and chief technology officer of Field Medical. “Our mission has always been to unite scientific credibility with innovation. These findings mark an important milestone as we continue to evaluate pulsed field ablation for its potential to improve outcomes for patients with ventricular arrhythmias.”
Looking ahead, the company is evaluating additional applications of its FieldForce Ablation System beyond VT and expects to present initial feasibility findings in atrial fibrillation (AF) at a major scientific meeting in early 2026.
About FieldForce™ Ablation System
The FieldForce Ablation System features a single-point contact force PFA catheter with an innovative design utilizing proprietary FieldBending™ technology to deliver targeted, brief, high-intensity electric fields. This next-generation PFA technology was designed to deliver both precise targeted lesions and large volume transmural lesions in the ventricle.
About Field Medical®, Inc.
Founded in 2022, Field Medical is a clinical-stage medical technology company committed to advancing pulsed field ablation (PFA) solutions for complex cardiac arrhythmias. Its FieldForce Ablation System integrates a focal catheter design with proprietary FieldBending energy designed to safely deliver efficient, precise ablation with the goal of improving outcomes in ventricular and atrial arrhythmia treatment. In 2024, Field Medical earned Breakthrough Device Designation and gained entry into the FDA TAP Pilot Program for its ventricular tachycardia indication.
For more information, visit www.fieldmedicalinc.com and follow us on LinkedInX, and YouTube.
The FieldForce™ Ablation System is an investigational device and is limited by federal (or United States) law to investigational use.
Source:
Reddy VY, et al. High-Voltage Focal Pulsed Field Ablation to Treat Scar-Related Ventricular Tachycardia: The First-in-Human VCAS Trial. Circulation. Published online ahead of print October 10, 2025. doi:10.1161/CIRCULATIONAHA.125.077025
CONTACT: Holly Windler, 619.929.1275, [email protected]
Photo – https://mma.prnewswire.com/media/2792975/Field_Medical_FieldForce__Ablation_System_Next_generation_PFA.jpg
Logo – https://mma.prnewswire.com/media/2574173/Field_Medical_Logo_Block_WhiteOnBlack_Logo.jpg

Findings presented at the International VT Symposium and published simultaneously in Circulation.
CARDIFF-BY-THE-SEA, Calif. and PHILADELPHIA, Oct. 10, 2025 /PRNewswire/ — Field Medical, Inc. today announced that
New Brunswick, New Jersey (October 10, 2025) – Johnson & Johnson (NYSE: JNJ) today announced that it has received notice of an unsolicited mini-tender offer by Tutanota LLC, a limited liability company established pursuant to the laws of the Island of Nevis to purchase up to 500,000 shares of Johnson & Johnson common stock at a price of $145.00 per share in cash. Tutanota’s offer price of $145.00 per share was well below the closing price of Johnson & Johnson common stock on September 26, 2025, the last full trading day prior to the date of the offer. Moreover, the offer is conditioned on, among other things, the closing price per share of Johnson & Johnson common stock exceeding $145.00 on the last trading day before the offer expires. This means that unless this condition is waived by Tutanota, Johnson & Johnson shareholders who tender their shares in the offer will receive a below-market price. Tutanota further states in its offering documents that it expects to extend the offer for successive periods of 45 to 180 days until the market price of the shares exceeds the offer price. The offer is for approximately 0.0207% of the shares of Johnson & Johnson common stock outstanding as of the September 29, 2025 offer date.
Johnson & Johnson is not associated in any way with Tutanota LLC or its unsolicited mini-tender offer and recommends that shareholders do not tender their shares in response to Tutanota’s offer because the offer is at a price below the current market price for Johnson & Johnson’s shares and subject to numerous conditions.
Tutanota has made many similar mini-tender offers for shares of other companies. Mini-tender offers seek to acquire less than 5 percent of a company’s shares outstanding, thereby avoiding many disclosure and procedural requirements of the U.S. Securities and Exchange Commission (SEC) that would otherwise apply. As a result, mini-tender offers do not provide investors with the same level of protections as provided for larger tender offers under U.S. securities laws.
The SEC has cautioned investors that some bidders making mini-tender offers at below-market prices are “hoping that they will catch investors off guard if the investors do not compare the offer price to the current market price.” More on the SEC’s guidance to investors on mini-tender offers is available at
https://www.sec.gov/rules-regulations/2000/07/commission-guidance-mini-tender-offers-limited-partnership-tender-offers.
Johnson & Johnson urges investors to obtain current market quotations for their shares, to consult with their broker or financial advisor and to exercise caution with respect to Tutanota’s offer. Johnson & Johnson recommends that shareholders who have not responded to Tutanota’s offer take no action. The offer is currently scheduled to expire at 5:00 p.m., New York City time, on Wednesday, October 29, 2025, unless extended or earlier terminated.
Johnson & Johnson encourages brokers and dealers, as well as other market participants, to review the SEC’s letter regarding broker-dealer mini-tender offer dissemination and disclosure at
www.sec.gov/divisions/marketreg/minitenders/sia072401.htm.
About Johnson & Johnson
At Johnson & Johnson, we believe health is everything. Our strength in healthcare innovation empowers us to build a world where complex diseases are prevented, treated, and cured, where treatments are smarter and less invasive, and solutions are personal. Through our expertise in Innovative Medicine and MedTech, we are uniquely positioned to innovate across the full spectrum of healthcare solutions today to deliver the breakthroughs of tomorrow, and profoundly impact health for humanity. Learn more at
www.jnj.com.

Written by BENJAMIN PERCY
Art and Cover by GEOFF SHAW
On Sale 2/11
DANGEROUS. UNHINGED. DEADLY. Deadpool is a man on the brink, and that means the jobs are deadlier, the stakes are higher, and the humor is darker than ever before. But Wade Wilson…
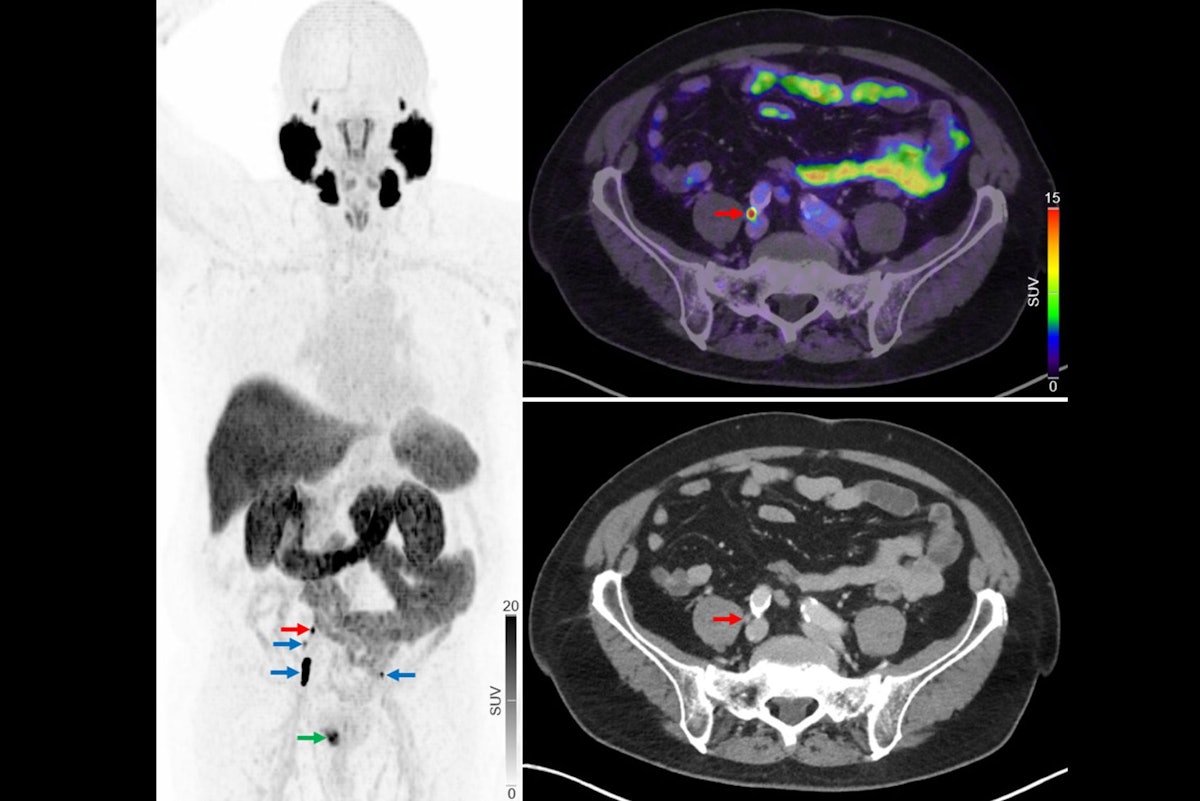
Replacing F-18 sodium fluoride (NaF) PET/CT imaging with F-18 prostate-specific membrane antigen (PSMA) PET/CT can improve treatment decisions among men with newly diagnosed prostate cancer, researchers in Denmark have reported.
Among 160…

Air pollution’s reach extends far beyond the lungs. Mounting evidence now ties dirty air not only to heart disease and respiratory illness but also to deeper metabolic disorders like insulin resistance and type 2 diabetes.
A new study led by…

After 15 years, four records and a buzz-making barrage of shows, tours and festivals, the moody, multifaceted music of north London’s Wolf Alice is huge in the U.K., thanks to uniquely seductive soundscapes, visceral live shows and a…

A new Gordon Ramsay-produced docuseries that started Friday offers the rare look into the ultimate chef quest: the pursuit of a…