- Australia lose Inglis, Zampa for first India ODI Reuters
- Zampa, Inglis to miss Perth ODI against India, Kuhnemann, Philippe called up ESPNcricinfo
- Inglis, Zampa to miss opening ODI against India ICC
- AUS vs IND 2025: ‘Everyone loves playing…
Blog
-
Australia lose Inglis, Zampa for first India ODI – Reuters
-

Justin Trudeau’s secret effort to win over Katy Perry uncovered
Justin Trudeau’s move to win over Katy Perry laid bare Justin Trudeau reportedly has been “pursuing” the popstar since their first meeting.
Following the couple’s recent PDA packed sighting, sources revealed to…
Continue Reading
-
LEQEMBI® IQLIK™(lecanemab-irmb) Subcutaneous Autoinjector Named to TIME’s “Best Inventions of 2025” – Biogen
- LEQEMBI® IQLIK™(lecanemab-irmb) Subcutaneous Autoinjector Named to TIME’s “Best Inventions of 2025” Biogen
- How Leqembi’s At-Home Injection Launch at Eisai (TSE:4523) Has Changed Its Investment Story simplywall.st
- Leqembi® Iqlik™ (lecanemab-irmb) maintenance treatment launched in the U.S. Yahoo Finance
- Eisai and Biogen’s LEQEMBI IQLIK™ Named One of TIME’s “Best Inventions of 2025” for Alzheimer’s Treatment Quiver Quantitative
- Gilead’s Yeztugo, Eisai’s Leqembi Iqlik, Vertex’s Journavx among 2025’s best inventions: Time Fierce Pharma
Continue Reading
-
Kate and Wills’ fresh start at their ‘forever home’: Why they have fast-tracked their move to house they will never leave – even when he becomes King
The Prince and Princess of Wales are hoping their fresh start will begin with a bang – moving into their new Windsor home by Bonfire Night.
The couple had initially planned to quit Adelaide Cottage on the royal Windsor estate and be in…
Continue Reading
-
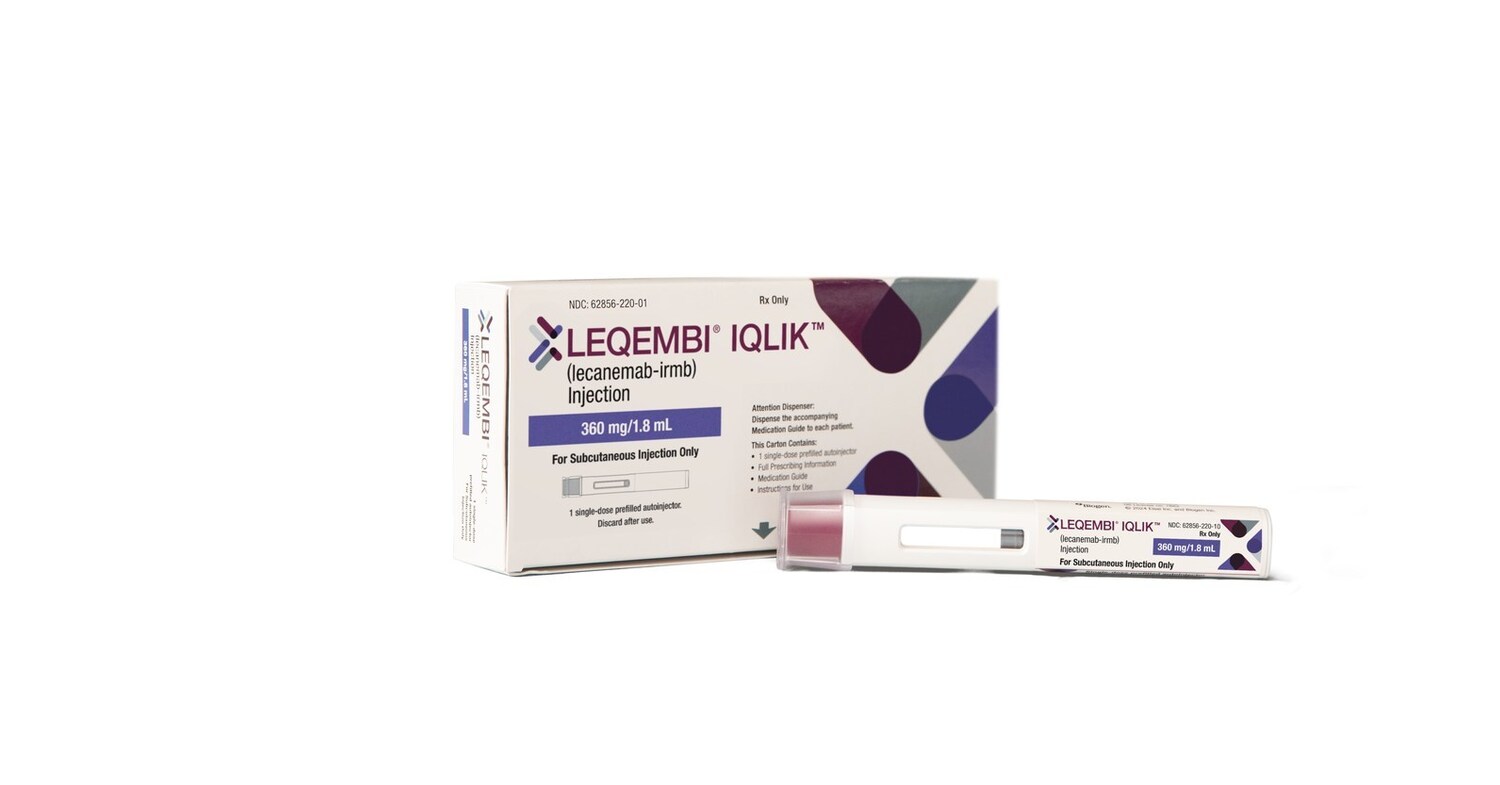
LEQEMBI® IQLIK™(lecanemab-irmb) Subcutaneous Autoinjector Named to TIME’s “Best Inventions of 2025”
TOKYO and CAMBRIDGE, Mass., Oct. 13, 2025 /PRNewswire/ — Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) and Biogen Inc. (Nasdaq: BIIB, Headquarters: Cambridge, Massachusetts, CEO: Christopher A. Viehbacher, “Biogen”) announced today that LEQEMBI® IQLIK™, a subcutaneous autoinjector formulation of lecanemab (generic name), for the treatment of Alzheimer’s disease (AD) has been selected by TIME as one of the “Best Inventions of 2025” in the Medical and Healthcare category.
LEQEMBI IQLIK product photo
TIME’s annual list of the Best Inventions features 300 extraordinary innovations changing lives. To compile the 2025 list, TIME solicited nominations from TIME editors and correspondents around the world, and through an online application process, paying special attention to growing fields such as health care and AI. TIME then evaluated each contender on a number of key factors, including originality, efficacy, ambition, and impact. For more information, please visit time.com/collections/best-inventions-2025/.
LEQEMBI IQLIK is the first and only anti-amyloid treatment to offer an at-home injection to help patients and their care partners continue to slow disease progression following the 18-month initial treatment period. The treatment was approved in the U.S. in August 2025 and launched on October 6. LEQEMBI IQLIK offers patients and their care partners the potential to shorten administration time (approximate injection time of 15 seconds), providing an option to continue treatment without having to worry about visiting an infusion center. Moreover, it has the potential to reduce healthcare resources associated with intravenous (IV) maintenance dosing, such as preparation for infusion and nurse monitoring, while increasing infusion capacity for new eligible patients to begin initiation treatment and streamlining the overall AD treatment pathway.
LEQEMBI, recognized as one of TIME’s “Best Inventions of 2023,” is the first approved anti-amyloid treatment for AD shown to slow disease progression and cognitive and functional decline in adults with Mild Cognitive Impairment (MCI) or mild dementia stage of disease (collectively referred to as early AD). LEQEMBI has been approved in 50 countries and is under regulatory review in 10 countries. In early September, Eisai initiated a rolling Supplemental Biologics License Application (sBLA) to the U.S. FDA for LEQEMBI IQLIK as a subcutaneous starting dose for the treatment of early AD under Fast Track Status.
Eisai serves as the lead of LEQEMBI development and regulatory submissions globally with Eisai and Biogen co-commercializing and co-promoting the product and Eisai having final decision-making authority.
INDICATION
LEQEMBI® is indicated for the treatment of Alzheimer’s disease (AD). Treatment with LEQEMBI should be initiated in patients with mild cognitive impairment (MCI) or mild dementia stage of disease, the population in which treatment was initiated in clinical trials.IMPORTANT SAFETY INFORMATION
WARNING: AMYLOID-RELATED IMAGING ABNORMALITIES (ARIA)
•
Monoclonal antibodies directed against aggregated forms of beta amyloid, including LEQEMBI, can cause ARIA, characterized as ARIA with edema (ARIA-E) and ARIA with hemosiderin deposition (ARIA-H). Incidence and timing of ARIA vary among treatments. ARIA usually occurs early in treatment and is usually asymptomatic, although serious and life-threatening events, including seizure and status epilepticus, can occur. ARIA can be fatal. Serious intracerebral hemorrhages (ICH) >1 cm, some of which have been fatal, have been observed with this class of medications. Because ARIA-E can cause focal neurologic deficits that can mimic an ischemic stroke, consider whether such symptoms could be due to ARIA-E before giving thrombolytic therapy to a patient being treated with LEQEMBI.
•
Apolipoprotein E ε4 (ApoE ε4) Homozygotes: Patients who are ApoE ε4 homozygotes (~15% of patients with AD) treated with this class of medications have a higher incidence of ARIA, including symptomatic, serious, and severe radiographic ARIA, compared to heterozygotes and noncarriers. Testing for ApoE ε4 status should be performed prior to initiation of treatment to inform the risk of developing ARIA. Prior to testing, prescribers should discuss with patients the risk of ARIA across genotypes and the implications of genetic testing results. Prescribers should inform patients that if genotype testing is not performed, they can still be treated with LEQEMBI; however, it cannot be determined if they are ApoE ε4 homozygotes and at higher risk for ARIA.
•
Consider the benefit of LEQEMBI for the treatment of AD and the potential risk of serious ARIA events when deciding to initiate treatment with LEQEMBI.
C ONTRAINDICATION
Contraindicated in patients with serious hypersensitivity to lecanemab-irmb or to any of the excipients. Reactions have included angioedema and anaphylaxis.WARNINGS AND PRECAUTIONS
AMYLOID-RELATED IMAGING ABNORMALITIES
Medications in this class, including LEQEMBI, can cause ARIA-E, which can be observed on MRI as brain edema or sulcal effusions, and ARIA-H, which includes microhemorrhage and superficial siderosis. ARIA can occur spontaneously in patients with AD, particularly in patients with MRI findings suggestive of cerebral amyloid angiopathy (CAA), such as pretreatment microhemorrhage or superficial siderosis. ARIA-H generally occurs with ARIA-E. Reported ARIA symptoms may include headache, confusion, visual changes, dizziness, nausea, and gait difficulty. Focal neurologic deficits may also occur. Symptoms usually resolve over time.Incidence of ARIA
Symptomatic ARIA occurred in 3% and serious ARIA symptoms in 0.7% with LEQEMBI. Clinical ARIA symptoms resolved in 79% of patients during the period of observation. ARIA, including asymptomatic radiographic events, was observed: LEQEMBI, 21%; placebo, 9%. ARIA-E was observed: LEQEMBI, 13%; placebo, 2%. ARIA-H was observed: LEQEMBI, 17%; placebo, 9%. No increase in isolated ARIA-H was observed for LEQEMBI vs placebo.Incidence of ICH
ICH >1 cm in diameter was reported in 0.7% with LEQEMBI vs 0.1% with placebo. Fatal events of ICH in patients taking LEQEMBI have been observed.Risk Factors of ARIA and ICH
ApoE ε4 Carrier Status
Of the patients taking LEQEMBI, 16% were ApoE ε4 homozygotes, 53% were heterozygotes, and 31% were noncarriers. With LEQEMBI, ARIA was higher in ApoE ε4 homozygotes (LEQEMBI: 45%; placebo: 22%) than in heterozygotes (LEQEMBI: 19%; placebo: 9%) and noncarriers (LEQEMBI: 13%; placebo: 4%). Symptomatic ARIA-E occurred in 9% of ApoE ε4 homozygotes vs 2% of heterozygotes and 1% of noncarriers. Serious ARIA events occurred in 3% of ApoE ε4 homozygotes and in ~1% of heterozygotes and noncarriers. The recommendations on management of ARIA do not differ between ApoE ε4 carriers and noncarriers.Radiographic Findings of CAA
Neuroimaging findings that may indicate CAA include evidence of prior ICH, cerebral microhemorrhage, and cortical superficial siderosis. CAA has an increased risk for ICH. The presence of an ApoE ε4 allele is also associated with CAA.The baseline presence of at least 2 microhemorrhages or the presence of at least 1 area of superficial siderosis on MRI, which may be suggestive of CAA, have been identified as risk factors for ARIA. Patients were excluded from Clarity AD for the presence of >4 microhemorrhages and additional findings suggestive of CAA (prior cerebral hemorrhage >1 cm in greatest diameter, superficial siderosis, vasogenic edema) or other lesions (aneurysm, vascular malformation) that could potentially increase the risk of ICH.
Concomitant Antithrombotic or Thrombolytic Medication
In Clarity AD, baseline use of antithrombotic medication (aspirin, other antiplatelets, or anticoagulants) was allowed if the patient was on a stable dose. Most exposures were to aspirin. Antithrombotic medications did not increase the risk of ARIA with LEQEMBI. The incidence of ICH: 0.9% in patients taking LEQEMBI with a concomitant antithrombotic medication vs 0.6% with no antithrombotic and 2.5% in patients taking LEQEMBI with an anticoagulant alone or with antiplatelet medication such as aspirin vs none in patients receiving placebo.Fatal cerebral hemorrhage has occurred in 1 patient taking an anti-amyloid monoclonal antibody in the setting of focal neurologic symptoms of ARIA and the use of a thrombolytic agent.
Additional caution should be exercised when considering the administration of antithrombotics or a thrombolytic agent (e.g., tissue plasminogen activator) to a patient already being treated with LEQEMBI. Because ARIA-E can cause focal neurologic deficits that can mimic an ischemic stroke, treating clinicians should consider whether such symptoms could be due to ARIA-E before giving thrombolytic therapy in a patient being treated with LEQEMBI.
Caution should be exercised when considering the use of LEQEMBI in patients with factors that indicate an increased risk for ICH and, in particular, patients who need to be on anticoagulant therapy or patients with findings on MRI that are suggestive of CAA.
Radiographic Severity With LEQEMBI
Most ARIA-E radiographic events occurred within the first 7 doses, although ARIA can occur at any time, and patients can have >1 episode. Maximum radiographic severity of ARIA-E with LEQEMBI was mild in 4%, moderate in 7%, and severe in 1% of patients. Resolution on MRI occurred in 52% of ARIA-E patients by 12 weeks, 81% by 17 weeks, and 100% overall after detection. Maximum radiographic severity of ARIA-H microhemorrhage with LEQEMBI was mild in 9%, moderate in 2%, and severe in 3% of patients; superficial siderosis was mild in 4%, moderate in 1%, and severe in 0.4% of patients. With LEQEMBI, the rate of severe radiographic ARIA-E was highest in ApoE ε4 homozygotes (5%) vs heterozygotes (0.4%) or noncarriers (0%). With LEQEMBI, the rate of severe radiographic ARIA-H was highest in ApoE ε4 homozygotes (13.5%) vs heterozygotes (2.1%) or noncarriers (1.1%).Monitoring and Dose Management Guidelines
Baseline brain MRI and periodic monitoring with MRI are recommended. Enhanced clinical vigilance for ARIA is recommended during the first 14 weeks of treatment. Depending on ARIA-E and ARIA-H clinical symptoms and radiographic severity, use clinical judgment when considering whether to continue dosing or to temporarily or permanently discontinue LEQEMBI. If a patient experiences ARIA symptoms, clinical evaluation should be performed, including MRI if indicated. If ARIA is observed on MRI, careful clinical evaluation should be performed prior to continuing treatment.HYPERSENSITIVITY REACTIONS
Hypersensitivity reactions, including angioedema, bronchospasm, and anaphylaxis, have occurred with LEQEMBI. Promptly discontinue the infusion upon the first observation of any signs or symptoms consistent with a hypersensitivity reaction and initiate appropriate therapy.INFUSION-RELATED REACTIONS (IRR s)
IRRs were observed—LEQEMBI: 26%; placebo: 7%—and most cases with LEQEMBI (75%) occurred with the first infusion. IRRs were mostly mild (69%) or moderate (28%). Symptoms included fever and flu-like symptoms (chills, generalized aches, feeling shaky, and joint pain), nausea, vomiting, hypotension, hypertension, and oxygen desaturation.IRRs can occur during or after the completion of infusion. In the event of an IRR during the infusion, the infusion rate may be reduced or discontinued, and appropriate therapy initiated as clinically indicated. Consider prophylactic treatment prior to future infusions with antihistamines, acetaminophen, nonsteroidal anti-inflammatory drugs, or corticosteroids.
ADVERSE REACTIONS
- The most common adverse reactions reported in ≥5% with LEQEMBI infusion every 2 weeks and ≥2% higher than placebo were IRRs (LEQEMBI: 26%; placebo: 7%), ARIA-H (LEQEMBI: 14%; placebo: 8%), ARIA-E (LEQEMBI: 13%; placebo: 2%), headache (LEQEMBI: 11%; placebo: 8%), superficial siderosis of central nervous system (LEQEMBI: 6%; placebo: 3%), rash (LEQEMBI: 6%; placebo: 4%), and nausea/vomiting (LEQEMBI: 6%; placebo: 4%)
- Safety profile of LEQEMBI IQLIK for maintenance treatment was similar to LEQEMBI infusion. Patients who received LEQEMBI IQLIK experienced localized and systemic (less frequent) injection-related reactions (mild to moderate in severity)
LEQEMBI (lecanemab-irmb) is available:
- Intravenous infusion: 100 mg/mL
- Subcutaneous injection: 200 mg/mL
Please see full Prescribing Information for LEQEMBI, including Boxed WARNING.
MEDIA CONTACTS
Eisai Co., Ltd.
Public Relations Department
TEL: +81 (0)3-3817-5120
Eisai Europe, Ltd.
EMEA Communications Department
+44 (0) 797 487 9419
[email protected]
Eisai Inc. (U.S.)
Libby Holman
+1-201-753-1945
[email protected]
Biogen Inc.
Madeleine Shin
+1-781-464-3260
[email protected]
INVESTOR CONTACTS
Eisai Co., Ltd.
Investor Relations Department
TEL: +81 (0) 3-3817-5122
Biogen Inc.
Tim Power
+ 1-781-464-2442
[email protected]
Notes to Editors
- About lecanemab (generic name, brand name: LEQEMBI ® )
Lecanemab is the result of a strategic research alliance between Eisai and BioArctic. It is a humanized immunoglobulin gamma (IgG1) monoclonal antibody directed against aggregated soluble (protofibril) and insoluble forms of amyloid-beta (Aβ).Lecanemab has been approved in 50 countries and is under regulatory review in 10 countries. The supplemental Biologics License Application (sBLA) for intravenous (IV) maintenance dosing of the treatment was approved in the U.S.in January 2025 and China in September 2025, and applications have been filed in 5 countries and regions.
LEQEMBI’s approvals in these countries was based on Phase 3 data from Eisai’s, global Clarity AD clinical trial, in which it met its primary endpoint and all key secondary endpoints with statistically significant results. The primary endpoint was the global cognitive and functional scale, Clinical Dementia Rating Sum of Boxes (CDR-SB). In the Clarity AD clinical trial, treatment with lecanemab reduced clinical decline on CDR-SB by 27% at 18 months compared to placebo. The mean CDR-SB score at baseline was approximately 3.2 in both groups. The adjusted least-squares mean change from baseline at 18 months was 1.21 with lecanemab and 1.66 with placebo (difference, −0.45; 95% confidence interval [CI], −0.67 to −0.23; P<0.001). In addition, the secondary endpoint from the AD Cooperative Study-Activities of Daily Living Scale for Mild Cognitive Impairment (ADCS-MCI-ADL), which measures information provided by people caring for patients with AD, noted a statistically significant benefit of 37% compared to placebo. The adjusted mean change from baseline at 18 months in the ADCS-MCI-ADL score was −3.5 in the lecanemab group and −5.5 in the placebo group (difference, 2.0; 95% CI, 1.2 to 2.8; P<0.001). The ADCS MCI-ADL assesses the ability of patients to function independently, including being able to dress, feed themselves and participate in community activities. The most common adverse events (>10%) in the lecanemab group were infusion reactions, ARIA-H (combined cerebral microhemorrhages, cerebral macrohemorrhages, and superficial siderosis), ARIA-E (edema/effusion), headache, and fall.
The subcutaneous maintenance dosing approval is based on LEQEMBI subcutaneous (SC) sub-studies of the Phase 3 Clarity AD open-label extension (OLE) trial in individuals with early AD which evaluated a range of subcutaneous doses. Data shows that transitioning to the weekly LEQEMBI IQLIKTM autoinjector after 18 months of the initiation dose (10 mg/kg IV every two weeks) maintains clinical and biomarker benefits comparable to continued IV dosing. The safety of LEQEMBI IQLIK autoinjector was studied in over 600 patients at a range of doses as part of the Clarity AD OLE. Across all subcutaneous doses, the safety profile was similar to that of the IV maintenance treatment with one key difference: systemic reactions were much less common with subcutaneous dosing—less than 1% compared to approximately 26% with IV infusions. ARIA rates in patients who received a weekly 360 mg subcutaneous maintenance dose were similar to ARIA rates reported in patients who continued with the IV dose after 18 months and are similar to the background rates of ARIA in patients without treatment.
Since July 2020, the Phase 3 clinical study (AHEAD 3-45) for individuals with preclinical AD, meaning they are clinically normal and have intermediate or elevated levels of amyloid in their brains, is ongoing. AHEAD 3-45 is conducted as a public-private partnership between the Alzheimer’s Clinical Trial Consortium that provides the infrastructure for academic clinical trials in AD and related dementias in the U.S, funded by the National Institute on Aging, part of the National Institutes of Health, Eisai, and Biogen. Since January 2022, the Tau NexGen clinical study for Dominantly Inherited AD (DIAD), that is conducted by Dominantly Inherited Alzheimer Network Trials Unit (DIAN-TU), led by Washington University School of Medicine in St. Louis, is ongoing and includes lecanemab as the backbone anti-amyloid therapy.
- About the Collaboration between Eisai and Biogen for AD
Eisai and Biogen have been collaborating on the joint development and commercialization of AD treatments since 2014. Eisai serves as the lead of lecanemab development and regulatory submissions globally with both companies co-commercializing and co-promoting the product and Eisai having final decision-making authority. - About the Collaboration between Eisai and BioArctic for AD
Since 2005, Eisai and BioArctic have had a long-term collaboration regarding the development and commercialization of AD treatments. Eisai obtained the global rights to study, develop, manufacture and market lecanemab for the treatment of AD pursuant to an agreement with BioArctic in December 2007. The development and commercialization agreement on the antibody lecanemab back-up was signed in May 2015. - About Eisai Co., Ltd.
Eisai’s Corporate Concept is “to give first thought to patients and people in the daily living domain, and to increase the benefits that health care provides.” Under this Concept (also known as human health care (hhc) Concept), we aim to effectively achieve social good in the form of relieving anxiety over health and reducing health disparities. With a global network of R&D facilities, manufacturing sites and marketing subsidiaries, we strive to create and deliver innovative products to target diseases with high unmet medical needs, with a particular focus in our strategic areas of Neurology and Oncology.In addition, we demonstrate our commitment to the elimination of neglected tropical diseases (NTDs), which is a target (3.3) of the United Nations Sustainable Development Goals (SDGs), by working on various activities together with global partners.
For more information about Eisai, please visit www.eisai.com (for global headquarters: Eisai Co., Ltd.), and connect with us on X, LinkedIn and Facebook. The website and social media channels are intended for audiences outside of the UK and Europe. For audiences based in the UK and Europe, please visit www.eisai.eu and Eisai EMEA LinkedIn.
- About Biogen
Founded in 1978, Biogen is a leading biotechnology company that pioneers innovative science to deliver new medicines to transform patient’s lives and to create value for shareholders and our communities. We apply deep understanding of human biology and leverage different modalities to advance first-in-class treatments or therapies that deliver superior outcomes. Our approach is to take bold risks, balanced with return on investment to deliver long-term growth.The company routinely posts information that may be important to investors on its website at www.biogen.com. Follow Biogen on social media – Facebook, LinkedIn, X, YouTube.
Biogen Safe Harbor
This news release contains forward-looking statements, including about the potential clinical effects of lecanemab; the potential benefits, safety and efficacy of lecanemab; potential regulatory discussions, submissions and approvals and the timing thereof including for lecanemab-irmb (LEQEMBI IQLIK); the potential to streamline the Alzheimer’s disease treatment pathway; the anticipated benefits and potential of Biogen’s collaboration arrangements with Eisai; the potential of Biogen’s commercial business and pipeline programs, including lecanemab; and risks and uncertainties associated with drug development and commercialization. These forward-looking statements may be accompanied by such words as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “forecast,” “goal,” “guidance,” “hope,” “intend,” “may,” “objective,” “plan,” “possible,” “potential,” “predict,” “project,” “prospect,” “should,” “target,” “will,” “would,” and other words and terms of similar meaning. Drug development and commercialization involve a high degree of risk, and only a small number of research and development programs result in commercialization of a product. Results in early-stage clinical trials may not be indicative of full results or results from later stage or larger scale clinical trials and do not ensure regulatory approval. You should not place undue reliance on these statements. Given their forward-looking nature, these statements involve substantial risks and uncertainties that may be based on inaccurate assumptions and could cause actual results to differ materially from those reflected in such statements. These forward-looking statements are based on management’s current beliefs and assumptions and on information currently available to management. Given their nature, we cannot assure that any outcome expressed in these forward-looking statements will be realized in whole or in part. We caution that these statements are subject to risks and uncertainties, many of which are outside of our control and could cause future events or results to be materially different from those stated or implied in this document, including, among others, uncertainty of long-term success in developing, licensing, or acquiring other product candidates or additional indications for existing products; expectations, plans and prospects relating to product approvals, approvals of additional indications for our existing products, sales, pricing, growth, reimbursement and launch of our marketed and pipeline products; our ability to effectively implement our corporate strategy; the successful execution of our strategic and growth initiatives, including acquisitions; the risk that positive results in a clinical trial may not be replicated in subsequent or confirmatory trials or success in early stage clinical trials may not be predictive of results in later stage or large scale clinical trials or trials in other potential indications; risks associated with clinical trials, including our ability to adequately manage clinical activities, unexpected concerns that may arise from additional data or analysis obtained during clinical trials, regulatory authorities may require additional information or further studies, or may fail to approve or may delay approval of our drug candidates; the occurrence of adverse safety events, restrictions on use with our products, or product liability claims; and any other risks and uncertainties that are described in other reports we have filed with the U.S. Securities and Exchange Commission.These statements speak only as of the date of this press release and are based on information and estimates available to us at this time. Should known or unknown risks or uncertainties materialize or should underlying assumptions prove inaccurate, actual results could vary materially from past results and those anticipated, estimated or projected. Investors are cautioned not to put undue reliance on forward-looking statements. A further list and description of risks, uncertainties and other matters can be found in our Annual Report on Form 10-K for the fiscal year ended December 31, 2024 and in our subsequent reports on Form 10-Q and Form 10-K, in each case including in the sections thereof captioned “Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in our subsequent reports on Form 8-K. Except as required by law, we do not undertake any obligation to publicly update any forward-looking statements whether as a result of any new information, future events, changed circumstances or otherwise.
SOURCE Eisai Inc.
Continue Reading
-

Slack Is Transforming Slackbot Into an AI Assistant
Slackbot, the assistant within the team communication platform Slack, is getting AI enhancements and integrations with other AI chatbots to become more agentic, Slack said in a presentation at Dreamforce, a tech conference in San Francisco, on…
Continue Reading
-

Camavinga involved in France’s draw with Iceland
Camavinga played 75 minutes in France’s draw with Iceland. The visitors headed into half-time a goal down after Palsson opened the scoring. Nkunku and Mateta struck to turn the scores around after the break, but Hlynsson found a leveller with…
Continue Reading
-

Vivo unveils X300 series with 200MP Zeiss cameras and MediaTek Dimensity 9500 chipset · TechNode
Chinese tech firm Vivo on Monday launched its annual flagship, the Vivo X300 series, in Shanghai. Centered on the concept of a Zeiss 200MP imaging system, the lineup includes both a standard and a Pro model. Coinciding with the…
Continue Reading
-
Samsung expects highest quarterly profit in over three years, lifted by AI chip demand – Reuters
- Samsung expects highest quarterly profit in over three years, lifted by AI chip demand Reuters
- Stars align for Samsung to post its highest Q3 profit in three years SamMobile
- Samsung Electronics Announces Earnings Guidance for Third Quarter 2025 samsung.com
- Samsung Elec estimates best-ever revenue and 3-year high OP for Q3 aju press
- Samsung May Have Made a Ton of Money in Third Quarter SammyGuru
Continue Reading
-

‘I lost a friend of almost 40 years’: Nancy Meyers pays tribute to Diane Keaton | Film
Film-maker Nancy Meyers has paid tribute to the late Diane Keaton, her “friend of almost 40 years” and collaborator on celebrated comedies Something’s Gotta Give, Baby Boom and Father of the Bride.
On Monday, Meyers wrote on Instagram that…
Continue Reading