After a huge race weekend in Austin, another one awaits as the teams make the relatively short trip south to Mexico City. With 40 points covering the top three drivers in the championship and plenty of young drivers set to get track time, it’s…
Blog
-

Plants self-organize in a ‘hidden order,’ echoing pattern found across nature
Scientists have uncovered a “hidden order” in drylands across the planet, where plants follow disordered hyperuniformity — a layout that looks random and disorganized up close but adheres to a clear pattern when viewed from farther away.
The…
Continue Reading
-
Combined with chemotherapy and radiotherapy is effective in improving prognosis: a case of primary mediastinal embryonal carcinoma | Journal of Cardiothoracic Surgery
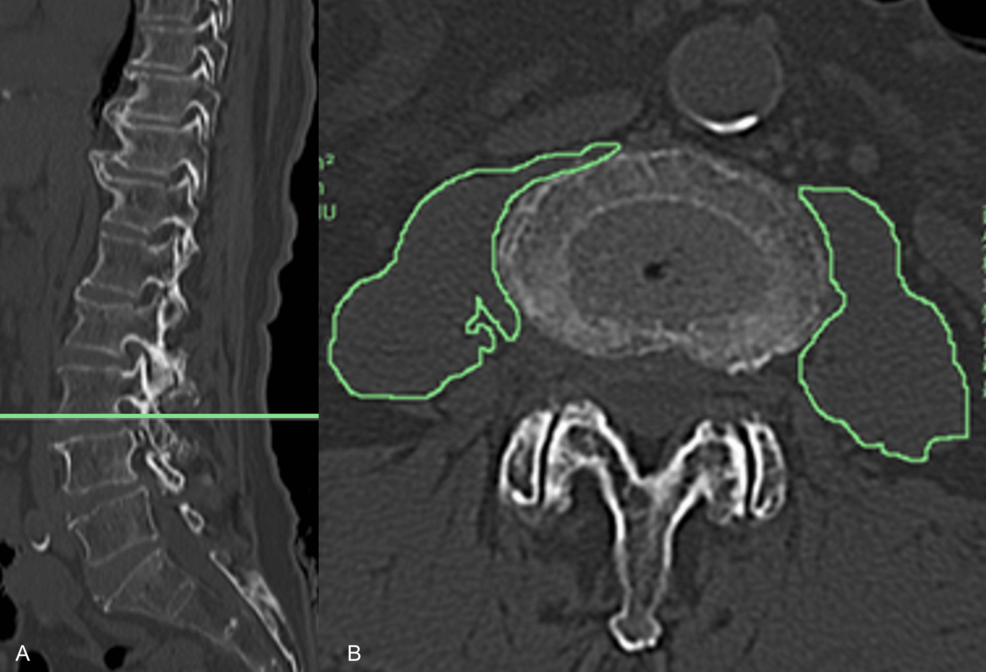
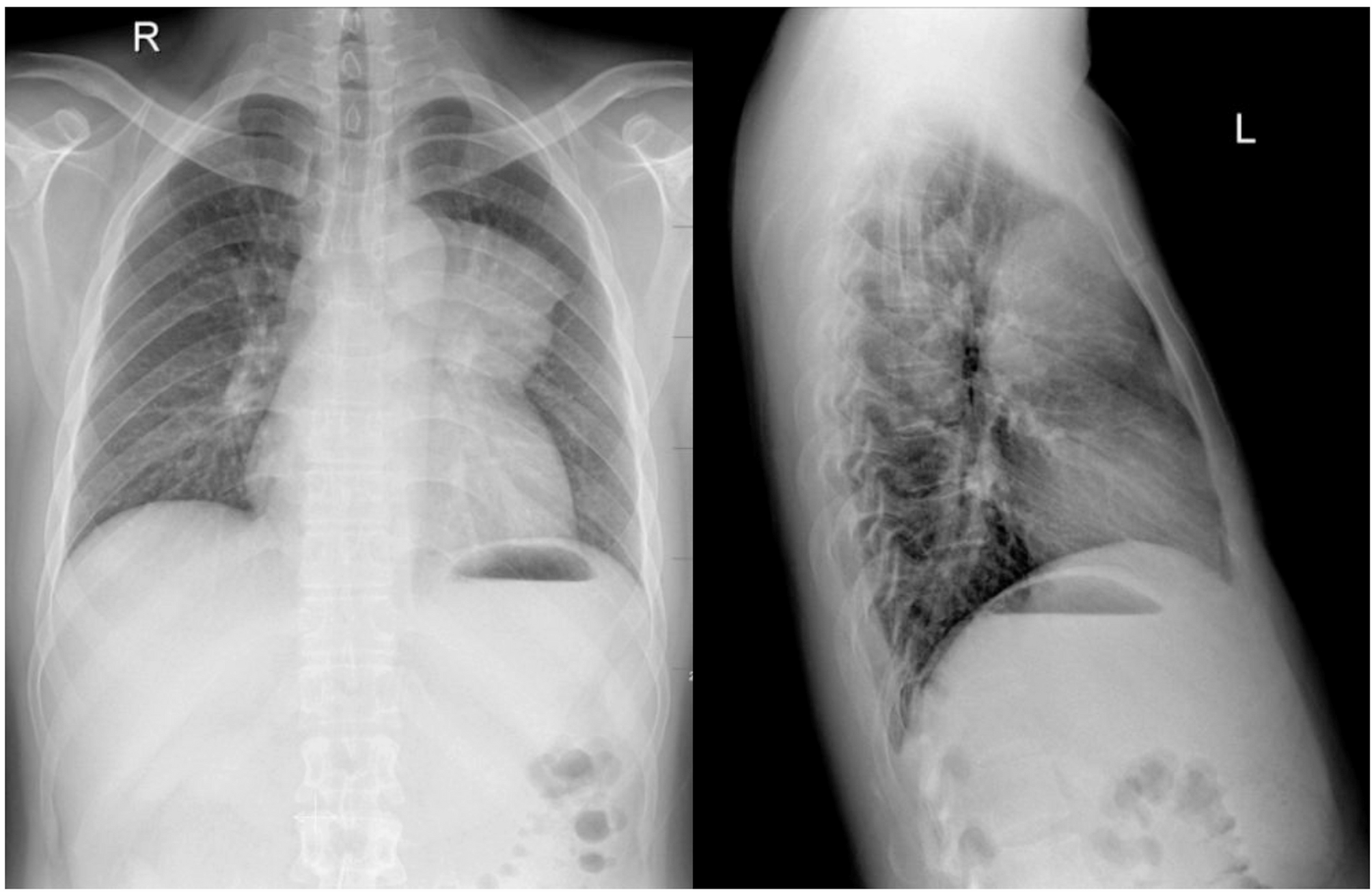
A 28-year-old man presented with spasmodic cough lasting for more than 1 month. Physical examination was normal. The chest radiography demonstrated a large, mass-like opacity located at the left side of the upper mediastinum(‘. 1). A…
Continue Reading
-
Disruption of low-frequency narrowband EEG microstate networks in Parkinson’s disease with mild cognitive impairment | Journal of NeuroEngineering and Rehabilitation
Participants and study design
This investigation enrolled a total of 67 individuals, comprising 47 individuals diagnosed with primary PD who were undergoing treatment at Beijing Rehabilitation Hospital, affiliated with Capital Medical University (25 males and 22 females), along with 20 individuals without any neurological conditions (healthy controls, HC) sourced from the local population, ensuring parity in gender and age. These controls had no past records of neurological impairments. Motor function was primarily appraised via the Movement Disorder Society Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRS III). Concurrently, cognitive abilities were assessed through the Mini-Mental State Examination (MMSE) as well as the Montreal Cognitive Assessment (MoCA) scales [22]. The MoCA is the most frequently used standard screening tool for PD-MCI, with a specificity of 82%, a sensitivity of 41%, and a diagnostic accuracy of 68% while maintaining clinical relevance [23]. From the MoCA results, two groups emerged among the 47 participants with PD: those with normal cognition (PD-NC, score > 25) and those with mild cognitive impairment (PD-MCI, score ≤ 25). With the approval of the Ethics Committee of Beijing Rehabilitation Hospital, Capital Medical University, we collected the data for this study (Approval Number: 2023bkky044). The data acquisition adhered rigorously to the ethical principles outlined in the Declaration of Helsinki. Before participating, all participants gave written informed consent.
EEG data acquisition and preprocessing
EEG data were recorded using a 30-channel electrode system (actiCAP snap, Brain Products GmbH, Gilching, Germany). The electrode positions included frontal electrodes (Fp1, Fz, F3, F4, F7, F8), fronto-central electrodes (FT9, FT10, FC5, FC6, FC1, FC2), central electrodes (C3, Cz, C4), parietal electrodes (CP5, CP6, CP1, CP2, Pz, P3, P4, P7, P8), and occipital electrodes (O1, Oz, O2). Participants sat relaxed with eyes closed in dimly lit rooms, maintaining wakefulness. The impedance of all electrodes was kept under 5 kΩ. The EEG signals were sampled at 1000 Hz for 15 min per individual. A rigorous, multi-stage preprocessing pipeline was applied to the raw EEG data to ensure high data quality and mitigate the constraints of the acquisition setup. Data were originally recorded using an actiCAP system with TP9 as the online reference. The data were first filtered using a zero-phase bandpass filter (0.5–45 Hz; Hamming-windowed Finite Impulse Response filter in EEGLAB) to attenuate low-frequency drifts and high-frequency noise. As the online reference (TP9) is suboptimal for Independent Component Analysis (ICA), the data were offline-referenced to the average of the bilateral mastoids (TP9 and TP10). This approach established a pragmatic and stable non-cephalic reference point for ICA, consistent with established practices in the field [24]. Subsequently, the data were decomposed into 30 independent components using the Infomax ICA algorithm. To ensure the validity of the decomposition and the accuracy of artifact rejection, a robust, semi-automated component classification strategy was employed. An initial, data-driven labeling was performed using ICLabel [25], followed by a rigorous manual review conducted by two experienced researchers. Components unambiguously identified as ocular (blinks, saccades), cardiac, or muscle artifacts (along with other categories) were rejected. A 5-minute continuous portion of EEG data per participant was selected for the purpose of microstate analysis. Due to the intermittent nature of artifacts, this 5-minute segment was often composed of multiple concatenated artifact-free intervals. The artifact-free EEG data were down-sampled to 125 Hz and then filtered for the broadband range (1–30 Hz). This broadband range was divided into four narrow bands based on their frequency ranges: the 1–4 Hz delta band, the 4–7 Hz theta band, the 7–13 Hz alpha band, and the 13–30 Hz beta band. Each narrow-band signal was further filtered using a zero-phase Finite Impulse Response filter (Hamming window) to ensure precise frequency isolation and temporal fidelity.
EEG spectral microstate analysis
The core principle of EEG microstate analysis is to segment the continuous EEG stream into a sequence of quasi-stable topographical maps, achieved by clustering the EEG data at specific time points [26]. The global field potential (GFP) quantifies the aggregate field strength of EEG signals at varying time points, as depicted in formula (1):
$$ \,GFP\left( t \right) = \sqrt {\frac{1}{N}\sum {\,_{{i = 1}}^{N} } (V_{i}^{{\prime \,}} (t))^{2} } $$
(1)
where \(\:{V}_{i}\left(t\right)\) denotes the potential of the \(\:i\)-th electrode at time\(\:\:t\), with \(\:N\) indicating the overall count of electrodes. Since the peak point of GFP typically coincides with the transition between microstates, it allows for the segmentation of microstate time points based on the GFP peak locations. GFP peaks were extracted with a minimum inter-peak distance of 10 ms and a maximum of 1000 peaks per subject. To mitigate noise, peaks with an amplitude exceeding one standard deviation above the mean GFP amplitude were excluded. Microstate templates were derived using a modified k-means clustering algorithm (modkmeans). The number of clusters (k) was fixed at 5, and the clustering procedure was repeated 50 times with random initializations to ensure solution stability. The optimal cluster set was selected based on the lowest cross-validation error, and the resulting maps were sorted by their global explained variance. Clustering iterations were limited to 1000 with a convergence threshold of 1e-6. Following template extraction, a back-fitting procedure was applied to assign a microstate label to each time point of the continuous EEG data for each subject. During this process, the polarity of the EEG signals was ignored to prevent label inversion. Subsequently, temporal smoothing was applied to remove microstate segments shorter than 30 ms, thereby improving the temporal continuity and physiological plausibility of the sequences. The refined, ongoing EEG data from each subject were sorted into their corresponding microstate types A, B, C, D, and E (abbreviated as MS-A, MS-B, MS-C, MS-D, and MS-E) by using the derived microstate type labels as templates. This analysis was performed on both the broadband data and data filtered into four classical frequency bands: delta, theta, alpha, and beta. For each of these five conditions, the preprocessed EEG data were independently matched against the set of microstate templates. From the resulting microstate sequences, several parameters were computed for each microstate class (Fig. 1A): (a) Coverage: The ratio of the duration a microstate is present to the total recording time. (b) Duration: Measures the length of each instance of a microstate. (c) Occurrence: The frequency of microstate occurrences throughout the recording period. (d) Transition Probability (TP): The chance that one microstate in the brain transforms into another.
Fig. 1 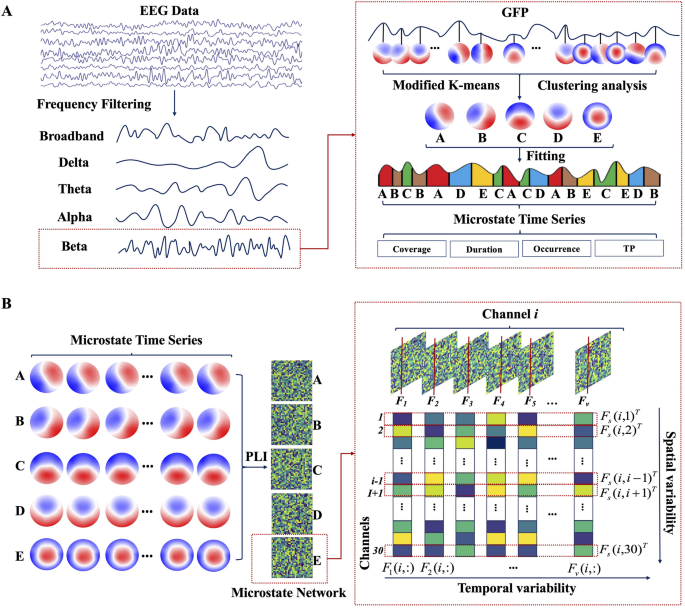
The scheme of the EEG microstate dynamics and spatiotemporal network analysis. A Steps for frequency–spectral microstate extraction, including bandpass filtering, global field power detection, microstate class identification, and microstate parameter analysis. B Steps for microstate-derived network analysis, including dynamic functional connectivity computation per microstate class by phase lag index, and quantification of temporal variability and spatial variability
EEG spectral microstate network analysis
The EEG microstate sequence for each individual was segmented into a series of discrete, non-overlapping intervals based on the assigned microstate labels (A-E). For each resulting microstate window, a functional connectivity network was constructed from 30 EEG electrodes using the Phase Lag Index (PLI). The PLI serves to measure the asymmetry level observed in the phase disparity between two signals [27]. Functional connectivity was estimated independently within each microstate segment, without concatenation or averaging across segments, resulting in a series of state-specific functional connectivity matrices. The segmentation algorithm adaptively defines the duration of each window, thereby avoiding arbitrary temporal partitioning and maintaining physiologically meaningful boundaries. Moreover, microstate segments, typically lasting between 60 and 300 ms, are considered quasi-stationary and have been empirically demonstrated to support reliable phase-based connectivity estimation [21]. Thus, the functional connectivity of paired EEG channels in the interval represented by the \(\:r\)-th microstate window was determined, as illustrated by formula (2),
$$ F_{r} \left( {i,j} \right) = \left| {~\left\langle {sign~\left[ {~sin\left( {\Delta \phi \left( {t_{l} } \right)} \right)~} \right]} \right\rangle ~} \right|l = 1,2,…,m_{r} ,i \ne j $$
(2)
where \(\:{F}_{r}(i,j)\) represents the functional connectivity between electrode channels \(\:i\) and \(\:j\) in the r-th microstate epoch. The notation \(\:\left\langle.\right\rangle\) is employed to denote the average value over that period. \(\:\Delta\varphi\:\left({t}_{l}\right)\) represents the phase differential determined at the \(\:l\)-th time instance, while \(\:{m}_{r}\) stands for the total number of time points within the \(\:r\)-th microstate epoch. By aggregating these PLI values for all pairs of EEG channels, the 30 × 30 adjacency matrix was constructed, which forms the brain network \(\:{F}_{r}\) for the \(\:r\)-th microstate window.
Subsequently, the functional connection networks corresponding to every distinct microstate category was divided into five different brain network sets (microstate networks A, B, C, D, and E). Utilizing these networks, the temporal and spatial variabilities were computed [21]. Temporal variability, by assessing the correlation between functional connectivity patterns of the same brain region across different time windows, captures the time-dependent changes of the functional architecture in specific brain areas, thereby providing insights into the temporal robustness of the region’s functional connectivity. The specific definition is shown in formula (3):
$$\:{T}_{i}=1-\frac{1}{v(v-1)}\sum\:_{p,q=1,p\ne\:q}^{v}\text{c}\text{o}\text{r}\text{r}\left({F}_{p}(i,:),{F}_{q}(i,:)\right)$$
(3)
where \(\:v\) is the number of microstate windows, \(\:{F}_{p}(i,:)\) indicates the functional arrangement of the \(\:i\)-th channel within the \(\:p\)-th microstate window, while \(\:corr({F}_{p}\left(i,:\right),{F}_{q}\left(i,:\right))\) calculates the correlation coefficient that measures the temporal similarity between two separate functional connectivity patterns. Spatial variability, by evaluating the correlation between the functional connectivity sequences of a particular brain region and those of other regions, reflects both the dynamic changes in local spatial functional connectivity and the spatial consistency of connectivity between regions. The definition is shown in formula (4),
$$\:{S}_{i}=1-\frac{1}{29\times\:28}\sum\:_{i,h=1,j\ne\:h\ne\:i}^{30}\text{c}\text{o}\text{r}\text{r}\left({F}_{s}(i,j),{F}_{s}(i,h)\right)$$
(4)
where \(\:corr({F}_{s}\left(i,j\right),{F}_{s}\left(i,h\right))\) denotes the correlation coefficient that quantifies the relationship between two different spatial functional connectivity patterns, \(\:{F}_{s}\left(i,j\right)\) and \(\:{F}_{s}\left(i,h\right)\). This metric is employed to assess the spatial consistency across all functional connectivity sequences associated with a particular scalp region (channel \(\:i\)) (Fig. 1B).
Clinical effect prediction
A feature set for classification was derived from microstate parameters, including coverage, duration, occurrence, and both temporal and spatial variability. These parameters were extracted across multiple frequency bands. To capture the spectral specificity of brain dynamics, these features were organized into distinct sets based on the following frequency bands: delta (1–4 Hz), theta (4–7 Hz), alpha (7–13 Hz), beta (13–30 Hz), and a broadband (1–30 Hz). A Random Forest classifier, implemented using a Bagging ensemble of decision trees, was trained for multi-class classification. To enhance model robustness and generalizability, hyperparameters (i.e., the number of trees, minimum leaf size, and maximum number of splits) were optimized via a Bayesian optimization algorithm. A nested cross-validation (CV) strategy was employed to prevent data leakage and overfitting. In this scheme, an inner 5-fold CV performed hyperparameter tuning, while an outer 5-fold CV provided an unbiased estimate of the model’s performance. To address potential class imbalance, class weights were set to be inversely proportional to class frequencies and applied during model training. The final model performance was determined by averaging the prediction metrics across the five outer folds of the nested CV. Classifier performance was comprehensively assessed using accuracy, sensitivity, and specificity. The results for each metric are reported as the mean ± standard deviation across these folds.
Statistical analysis
Data normality was evaluated by applying the Shapiro-Wilk test. The Mann-Whitney U test and independent t-tests were used to examine differences in demographic characteristics and clinical scores among the groups. Repeated measures Analysis of Variance was employed to evaluate group (PD-MCI, PD-NC, and HC) differences across narrowband and broadband microstate network metrics using four model specifications: (1) microstate parameters (3 groups × 3 parameters [coverage, duration, and occurrence] × 5 classes [MS-A, MS-B, MS-C, MS-D, and MS-E]), (2) transition probabilities (3 groups × 20 metrics [A to B/C/D/E, B to A/C/D/E, C to A/B/D/E, D to A/B/C/E, E to A/B/C/D]), (3) microstate network regional temporal or spatial variabilities (3 groups × 5 microstate levels × 30 channels), and (4) microstate network global temporal or spatial variabilities (3 groups × 5 classes [MS-A, MS-B, MS-C, MS-D, and MS-E]). Where significant main and interaction effects emerged (p < 0.05), post hoc analyses was conducted using Bonferroni-corrected paired-samples t-tests. Pearson correlations then quantified relationships between MoCA scores and group-discriminating features in PD subgroups (PD-MCI vs. PD-NC), with independent Bonferroni corrections applied to four metric families per frequency band: microstate parameters, transition probabilities, regional temporal or spatial variabilities, and global temporal or spatial variabilities.
Continue Reading
-
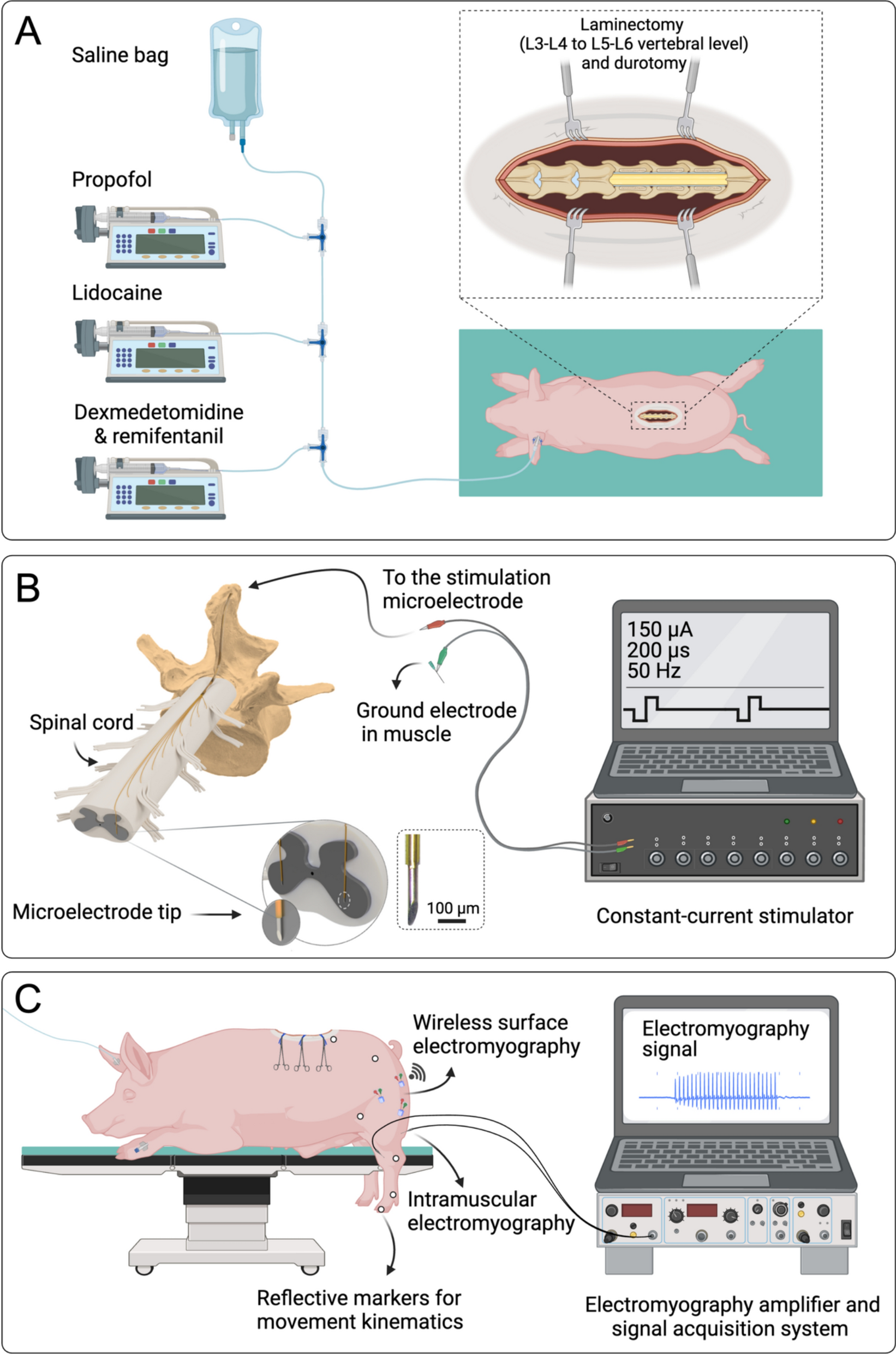
Functional motor mapping of domestic pig lumbar spinal cord using penetrating microelectrodes | Journal of NeuroEngineering and Rehabilitation
Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;10:282.
Google Scholar
National Spinal Cord Injury Statistical Center. Traumatic spinal cord injury facts and figures at a glance. Birmingham, AL: University of Alabama at Birmingham [Internet]. 2024; Available from: https://msktc.org/sites/default/files/Facts-and-Figures-2024-Eng-508.pdf
Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–83.
Google Scholar
Brown-Triolo DL, Roach MJ, Nelson K, Triolo R. Consumer perspectives on mobility: implications for neuroprosthesis design. Journal of Rehabilitation Research & Development. 2002;39(6).
Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377(9781):1938–47.
Google Scholar
Rowald A, Komi S, Demesmaeker R, Baaklini E, Hernandez-Charpak SD, Paoles E, et al. Activity-dependent spinal cord neuromodulation rapidly restores trunk and leg motor functions after complete paralysis. Nat Med. 2022;28(2):260–71.
Google Scholar
Gill ML, Grahn PJ, Calvert JS, Linde MB, Lavrov IA, Strommen JA, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med. 2018;24(11):1677–82.
Google Scholar
Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med. 2018;379(13):1244–50.
Google Scholar
Hofstoetter US, Perret I, Bayart A, Lackner P, Binder H, Freundl B, et al. Spinal motor mapping by epidural stimulation of lumbosacral posterior roots in humans. iScience. 2021. https://doi.org/10.1016/j.isci.2020.101930.
Google Scholar
Gorgey AS, Trainer R, Sutor TW, Goldsmith JA, Alazzam A, Goetz LL, et al. A case study of percutaneous epidural stimulation to enable motor control in two men after spinal cord injury. Nat Commun. 2023;14(1):2064.
Google Scholar
Mesbah S, Herrity A, Ugiliweneza B, Angeli C, Gerasimenko Y, Boakye M, et al. Neuroanatomical mapping of the lumbosacral spinal cord in individuals with chronic spinal cord injury. Brain Commun. 2023;5(1):fcac330.
Google Scholar
Mong ER, Kethireddy S, Staudt MD. Spinal cord stimulator paddle lead revision and replacement for misplaced or displaced electrodes. World Neurosurg. 2024. https://doi.org/10.1016/j.wneu.2024.03.154.
Google Scholar
Dombovy-Johnson ML, D’Souza RS, Ha CT, Hagedorn JM. Incidence and risk factors for spinal cord stimulator lead migration with or without loss of efficacy: a retrospective review of 91 consecutive thoracic lead implants. Neuromodulation Technol Neural Interface. 2022;25(5):731–7.
Alazzam AM, Ballance WB, Smith AC, Rejc E, Weber KA, Trainer R, et al. Peak slope ratio of the recruitment curves compared to muscle evoked potentials to optimize standing configurations with percutaneous epidural stimulation after spinal cord injury. J Clin Med. 2024;13(5):1344.
Google Scholar
Boakye M, Ball T, Dietz N, Sharma M, Angeli C, Rejc E, et al. Spinal cord epidural stimulation for motor and autonomic function recovery after chronic spinal cord injury: a case series and technical note. Surg Neurol Int. 2023. https://doi.org/10.25259/SNI_1074_2022.
Google Scholar
Rejc E, Angeli CA, Bryant N, Harkema SJ. Effects of stand and step training with epidural stimulation on motor function for standing in chronic complete paraplegics. J Neurotrauma. 2017;34(9):1787–802.
Google Scholar
Capogrosso M, Wenger N, Raspopovic S, Musienko P, Beauparlant J, Luciani LB, et al. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J Neurosci. 2013;33(49):19326–40.
Google Scholar
Greiner N, Barra B, Schiavone G, Lorach H, James N, Conti S, et al. Recruitment of upper-limb motoneurons with epidural electrical stimulation of the cervical spinal cord. Nat Commun. 2021;12(1):435.
Google Scholar
Choi EH, Gattas S, Brown NJ, Hong JD, Limbo JN, Chan AY, et al. Epidural electrical stimulation for spinal cord injury. Neural Regen Res. 2021;16(12):2367–75.
Google Scholar
Saigal R, Renzi C, Mushahwar VK. Intraspinal microstimulation generates functional movements after spinal-cord injury. IEEE Trans Neural Syst Rehabil Eng. 2004;12(4):430–40.
Google Scholar
Mushahwar VK, Gillard DM, Gauthier MJ, Prochazka A. Intraspinal micro stimulation generates locomotor-like and feedback-controlled movements. IEEE Trans Neural Syst Rehabil Eng. 2002;10(1):68–81.
Google Scholar
Sunshine MD, Cho FS, Lockwood DR, Fechko AS, Kasten MR, Moritz CT. Cervical intraspinal microstimulation evokes robust forelimb movements before and after injury. J Neural Eng. 2013;10(3):036001.
Google Scholar
Zimmermann JB, Seki K, Jackson A. Reanimating the arm and hand with intraspinal microstimulation. J Neural Eng. 2011;8(5):054001.
Google Scholar
Gaunt RA, Prochazka A, Mushahwar VK, Guevremont L, Ellaway PH. Intraspinal microstimulation excites multisegmental sensory afferents at lower stimulus levels than local α-motoneuron responses. J Neurophysiol. 2006;96(6):2995–3005.
Google Scholar
Zimmermann JB, Jackson A. Closed-loop control of spinal cord stimulation to restore hand function after paralysis. Front Neurosci. 2014;8:87.
Google Scholar
Bizzi E, Giszter SF, Loeb E, Mussa-Ivaldi FA, Saltiel P. Modular organization of motor behavior in the frog’s spinal cord. Trends Neurosci. 1995;18(10):442–6.
Google Scholar
Giszter SF. Spinal primitives and intra-spinal micro-stimulation (ISMS) based prostheses: a neurobiological perspective on the “known unknowns” in ISMS and future prospects. Front Neurosci. 2015;9:72.
Google Scholar
Moritz CT, Lucas TH, Perlmutter SI, Fetz EE. Forelimb movements and muscle responses evoked by microstimulation of cervical spinal cord in sedated monkeys. J Neurophysiol. 2007;97(1):110–20.
Google Scholar
Hachmann JT, Jeong JH, Grahn PJ, Mallory GW, Evertz LQ, Bieber AJ, et al. Large animal model for development of functional restoration paradigms using epidural and intraspinal stimulation. PLoS ONE. 2013;8(12):e81443.
Google Scholar
Sharpe AN, Jackson A. Upper-limb muscle responses to epidural, subdural and intraspinal stimulation of the cervical spinal cord. J Neural Eng. 2014;11(1):016005.
Google Scholar
Mushahwar VK, Collins DF, Prochazka A. Spinal cord microstimulation generates functional limb movements in chronically implanted cats. Exp Neurol. 2000;163(2):422–9.
Google Scholar
Tawakol O, Herman MD, Foxley S, Mushahwar VK, Towle VL, Troyk PR. In-vivo testing of a novel wireless intraspinal microstimulation interface for restoration of motor function following spinal cord injury. Artif Organs. 2024;48(3):263–73.
Google Scholar
Nashold B, Friedman H, Grimes J. Electrical stimulation of the conus medullaris to control bladder emptying in paraplegia: a ten-year review. Stereotact Funct Neurosurg. 1982;45(1–2):40–3.
Google Scholar
Nashold BS, Friedman H, Glenn JF, Grimes JH, Barry WF, Avery R. Electromicturition in paraplegia: implantation of a spinal neuroprosthesis. Arch Surg. 1972;104(2):195–202.
Google Scholar
Boergens KM, Tadić A, Hopper MS, McNamara I, Fell D, Sahasrabuddhe K, et al. Laser ablation of the pia mater for insertion of high-density microelectrode arrays in a translational sheep model. J Neural Eng. 2021;18(4):045008.
Rocca A, Lehner C, Wafula-Wekesa E, Luna E, Fernández-Cornejo V, Abarca-Olivas J, et al. Robot-assisted implantation of a microelectrode array in the occipital lobe as a visual prosthesis. J Neurosurg. 2023;140(4):1169–76.
Google Scholar
Roser F, Tatagiba M, Maier G. Spinal robotics: current applications and future perspectives. Neurosurgery. 2013;72:A12–8.
Zeng B, Meng F, Ding H, Wang G. A surgical robot with augmented reality visualization for stereoelectroencephalography electrode implantation. Int J Comput Assist Radiol Surg. 2017;12:1355–68.
Google Scholar
Prochazka A, Mushahwar V, Yakovenko S. Activation and coordination of spinal motoneuron pools after spinal cord injury. Prog Brain Res. 2002;137:109–24.
Google Scholar
Guevremont L, Renzi CG, Norton JA, Kowalczewski J, Saigal R, Mushahwar VK. Locomotor-related networks in the lumbosacral enlargement of the adult spinal cat: activation through intraspinal microstimulation. IEEE Trans Neural Syst Rehabil Eng. 2006;14(3):266–72.
Google Scholar
Toossi A, Bergin B, Marefatallah M, Parhizi B, Tyreman N, Everaert DG, et al. Comparative neuroanatomy of the lumbosacral spinal cord of the rat, cat, pig, monkey, and human. Sci Rep. 2021;11(1):1955.
Google Scholar
Lee JH, Jones CF, Okon EB, Anderson L, Tigchelaar S, Kooner P, et al. A novel porcine model of traumatic thoracic spinal cord injury. J Neurotrauma. 2013;30(3):142–59.
Google Scholar
Leonard AV, Menendez JY, Pat BM, Hadley MN, Floyd CL. Localization of the corticospinal tract within the porcine spinal cord: implications for experimental modeling of traumatic spinal cord injury. Neurosci Lett. 2017;648:1–7.
Google Scholar
Smith AC, Ahmed RU, Weber KA, Negahdar M, Gibson D, Boakye M, et al. Spinal cord lesion MRI and behavioral outcomes in a miniature pig model of spinal cord injury: exploring preclinical potential through an ad hoc comparison with human SCI. Spinal Cord Ser Cases. 2024;10(1):44.
Google Scholar
Ahmed RU, Knibbe CA, Wilkins F, Sherwood LC, Howland DR, Boakye M. Porcine spinal cord injury model for translational research across multiple functional systems. Exp Neurol. 2023;359:114267.
Google Scholar
Mirkiani S, Roszko DA, O’Sullivan CL, Faridi P, Hu DS, Fang D, et al. Overground gait kinematics and muscle activation patterns in the Yucatan mini pig. J Neural Eng. 2022;19(2):026009.
Boakye M, Morehouse J, Ethridge J, Burke DA, Khattar NK, Kumar C, et al. Treadmill-based gait kinematics in the Yucatan mini pig. J Neurotrauma. 2020;37(21):2277–91.
Google Scholar
Schiavone G, Wagner F, Fallegger F, Kang X, Vachicouras N, Barra B, et al. Long-term functionality of a soft electrode array for epidural spinal cord stimulation in a minipig model. In IEEE; 2018. p. 1432–5.
Hogan MK, Barber SM, Rao Z, Kondiles BR, Huang M, Steele WJ, et al. A wireless spinal stimulation system for ventral activation of the rat cervical spinal cord. Sci Rep. 2021;11(1):14900.
Google Scholar
Afridi AK, Steele AG, Martin C, Sayenko DG, Barber SM. Ventral epidural stimulation for motor recovery after spinal cord injury: illustrative case. Journal of Neurosurgery: Case Lessons. 2024;8(12).
Lin D, Lee JM, Wang C, Park HG, Lieber CM. Injectable ventral spinal stimulator evokes programmable and biomimetic hindlimb motion. Nano Lett. 2023;23(13):6184–92.
Google Scholar
Harland B, Kow CY, Svirskis D. Spinal intradural electrodes: opportunities, challenges and translation to the clinic. Neural Regen Res. 2023. https://doi.org/10.4103/1673-5374.380895.
Google Scholar
Olmsted ZT, Wu PB, Katouzian A, Dorsi MJ. Intrathecal placement of percutaneous spinal cord stimulation leads: illustrative cases. Journal of Neurosurgery: Case Lessons. 2024;8(13).
Chapman KB, Sayed D, Lamer T, Hunter C, Weisbein J, Patel KV, et al. Best practices for dorsal root ganglion stimulation for chronic pain: guidelines from the American Society of Pain and Neuroscience. J Pain Res. 2023;839–79.
King KW, Cusack WF, Nanivadekar AC, Ayers CA, Urbin M, Gaunt RA, et al. DRG microstimulation evokes postural responses in awake, standing felines. J Neural Eng. 2019;17(1):016014.
Google Scholar
Ayers CA, Fisher LE, Gaunt RA, Weber DJ. Microstimulation of the lumbar DRG recruits primary afferent neurons in localized regions of lower limb. J Neurophysiol. 2016;116(1):51–60.
Google Scholar
Dalrymple AN, Ting JE, Bose R, Trevathan JK, Nieuwoudt S, Lempka SF, et al. Stimulation of the dorsal root ganglion using an Injectrode®. J Neural Eng. 2021;18(5):056068.
Toossi A, Everaert DG, Perlmutter SI, Mushahwar VK. Functional organization of motor networks in the lumbosacral spinal cord of non-human primates. Sci Rep. 2019;9(1):13539.
Google Scholar
Toossi A, Everaert DG, Seres P, Jaremko JL, Robinson K, Kao CC, et al. Ultrasound-guided spinal stereotactic system for intraspinal implants. J Neurosurg Spine. 2018;29(3):292–305.
Google Scholar
Mirkiani S, O’Sullivan CL, Roszko DA, Faridi P, Hu DS, Everaert DG, et al. Safety of mapping the motor networks in the spinal cord using penetrating microelectrodes in Yucatan minipigs. Journal of Neurosurgery: Spine. 2024;1(aop):1–13.
Borrell JA, Frost SB, Peterson J, Nudo RJ. A 3d map of the hindlimb motor representation in the lumbar spinal cord in Sprague Dawley rats. J Neural Eng. 2016;14(1):016007.
Google Scholar
Mushahwar VK, Horch KW. Selective activation of muscle groups in the feline hindlimb through electrical microstimulation of the ventral lumbo-sacral spinal cord. IEEE Trans Rehabil Eng. 2000;8(1):11–21.
Google Scholar
Bamford JA, Todd KG, Mushahwar VK. The effects of intraspinal microstimulation on spinal cord tissue in the rat. Biomaterials. 2010;31(21):5552–63.
Google Scholar
Roszko DA, Mirkiani S, Tyreman N, Wilson D, Toossi A, Mushahwar VK. Laser-microfabricated polymer multielectrodes for intraspinal microstimulation. IEEE Trans Biomed Eng. 2022;70(1):354–65.
Google Scholar
Toossi A, Everaert DG, Uwiera RR, Hu DS, Robinson K, Gragasin FS, et al. Effect of anesthesia on motor responses evoked by spinal neural prostheses during intraoperative procedures. J Neural Eng. 2019;16(3):036003.
Google Scholar
Vanderhorst VG, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol. 1997;382(1):46–76.
Google Scholar
Tresch MC, Bizzi E. Responses to spinal microstimulation in the chronically spinalized rat and their relationship to spinal systems activated by low threshold cutaneous stimulation. Exp Brain Res. 1999;129:401–16.
Google Scholar
Capogrosso M, Milekovic T, Borton D, Wagner F, Moraud EM, Mignardot JB, et al. A brain–spine interface alleviating gait deficits after spinal cord injury in primates. Nature. 2016;539(7628):284–8.
Google Scholar
Sharrard W. The segmental innervation of the lower limb muscles in man: Arris and Gale lecture delivered at the Royal College of Surgeons of England on 2nd January 1964. Ann R Coll Surg Engl. 1964;35(2):106.
Google Scholar
Sharrard W. The distribution of the permanent paralysis in the lower limb in poliomyelitis: a clinical and pathological study. J Bone Joint Surgery British. 1955;37(4):540–58.
Yakovenko S, Mushahwar V, VanderHorst V, Holstege G, Prochazka A. Spatiotemporal activation of lumbosacral motoneurons in the locomotor step cycle. J Neurophysiol. 2002;87(3):1542–53.
Google Scholar
Bamford J, Putman C, Mushahwar V. Intraspinal microstimulation preferentially recruits fatigue-resistant muscle fibres and generates gradual force in rat. J Physiol. 2005;569(3):873–84.
Google Scholar
Holinski B, Mazurek K, Everaert D, Toossi A, Lucas-Osma A, Troyk P, et al. Intraspinal microstimulation produces over-ground walking in anesthetized cats. J Neural Eng. 2016;13(5):056016.
Google Scholar
Snow S, Horch KW, Mushahwar VK. Intraspinal microstimulation using cylindrical multielectrodes. IEEE Trans Biomed Eng. 2006;53(2):311–9.
Google Scholar
Lau B, Guevremont L, Mushahwar VK. Strategies for generating prolonged functional standing using intramuscular stimulation or intraspinal microstimulation. IEEE Trans Neural Syst Rehabil Eng. 2007;15(2):273–85.
Google Scholar
Vogelstein RJ, Tenore FV, Guevremont L, Etienne-Cummings R, Mushahwar VK. A silicon central pattern generator controls locomotion in vivo. IEEE Trans Biomed Circuits Syst. 2008;2(3):212–22.
Google Scholar
Dalrymple AN, Roszko DA, Sutton RS, Mushahwar VK. Pavlovian control of intraspinal microstimulation to produce over-ground walking. J Neural Eng. 2020;17(3):036002.
Google Scholar
Dalrymple AN, Everaert DG, Hu DS, Mushahwar VK. A speed-adaptive intraspinal microstimulation controller to restore weight-bearing stepping in a spinal cord hemisection model. J Neural Eng. 2018;15(5):056023.
Google Scholar
Fiori L, Castiglia SF, Chini G, Draicchio F, Sacco F, Serrao M, et al. The lower limb muscle co-activation map during human locomotion: from slow walking to running. Bioengineering. 2024;11(3):288.
Google Scholar
Cheng R, Sui Y, Sayenko D, Burdick JW. Motor control after human SCI through activation of muscle synergies under spinal cord stimulation. IEEE Trans Neural Syst Rehabil Eng. 2019;27(6):1331–40.
Google Scholar
Rasmussen S, Chan A, Goslow G Jr. The cat step cycle: electromyographic patterns for hindlimb muscles during posture and unrestrained locomotion. J Morphol. 1978;155(3):253–69.
Google Scholar
Aagaard P, Simonsen E, Andersen J, Magnusson S, Bojsen-Møller F, Dyhre-Poulsen P. Antagonist muscle coactivation during isokinetic knee extension. Scand J Med Sci Sports. 2000;10(2):58–67.
Google Scholar
Latash ML. Muscle coactivation: definitions, mechanisms, and functions. J Neurophysiol. 2018;120(1):88–104.
Google Scholar
Baratta R, Solomonow M, Zhou B, Letson D, Chuinard R, D’ambrosia R. Muscular coactivation: the role of the antagonist musculature in maintaining knee stability. Am J Sports Med. 1988;16(2):113–22.
Google Scholar
Tsuchida T, Ensini M, Morton S, Baldassare M, Edlund T, Jessell T, et al. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79(6):957–70.
Google Scholar
Tripodi M, Stepien AE, Arber S. Motor antagonism exposed by spatial segregation and timing of neurogenesis. Nature. 2011;479(7371):61–6.
Google Scholar
Takeoka A, Arber S. Functional local proprioceptive feedback circuits initiate and maintain locomotor recovery after spinal cord injury. Cell Rep. 2019;27(1):71–85.
Google Scholar
Ronzano R, Skarlatou S, Barriga BK, Bannatyne BA, Bhumbra GS, Foster JD, et al. Spinal premotor interneurons controlling antagonistic muscles are spatially intermingled. Elife. 2022;11:e81976.
Google Scholar
Levine AJ, Hinckley CA, Hilde KL, Driscoll SP, Poon TH, Montgomery JM, et al. Identification of a cellular node for motor control pathways. Nat Neurosci. 2014;17(4):586–93.
Google Scholar
Hanson TL, Diaz-Botia CA, Kharazia V, Maharbiz MM, Sabes PN. The “sewing machine” for minimally invasive neural recording. BioRxiv. 2019;578542.
Vomero M, Ciarpella F, Zucchini E, Kirsch M, Fadiga L, Stieglitz T, et al. On the longevity of flexible neural interfaces: establishing biostability of polyimide-based intracortical implants. Biomaterials. 2022;281:121372.
Google Scholar
Luan L, Wei X, Zhao Z, Siegel JJ, Potnis O, Tuppen CA, et al. Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Sci Adv. 2017;3(2):e1601966.
Google Scholar
Lee Y, Shin H, Lee D, Choi S, Cho I, Seo J. A lubricated nonimmunogenic neural probe for acute insertion trauma minimization and long-term signal recording. Adv Sci. 2021;8(15):2100231.
Google Scholar
Sridar S, Churchward MA, Mushahwar VK, Todd KG, Elias AL. Peptide modification of polyimide-insulated microwires: towards improved biocompatibility through reduced glial scarring. Acta Biomater. 2017;60:154–66.
Google Scholar
Toossi A, Everaert DG, Azar A, Dennison CR, Mushahwar VK. Mechanically stable intraspinal microstimulation implants for human translation. Ann Biomed Eng. 2017;45:681–94.
Google Scholar
Soloukey S, de Rooij JD, Osterthun R, Drenthen J, De Zeeuw CI, Huygen FJ, et al. The dorsal root ganglion as a novel neuromodulatory target to evoke strong and reproducible motor responses in chronic motor complete spinal cord injury: a case series of five patients. Neuromodul Technol Neural Interface. 2021;24(4):779–93.
Holinski BJ, Mazurek KA, Everaert DG, Stein RB, Mushahwar VK. Restoring stepping after spinal cord injury using intraspinal microstimulation and novel control strategies. In IEEE; 2011. p. 5798–801.
Kramer J, Liem L, Russo M, Smet I, Van Buyten JP, Huygen F. Lack of body positional effects on paresthesias when stimulating the dorsal root ganglion (DRG) in the treatment of chronic pain. Neuromodul Technol Neural Interface. 2015;18(1):50–7.
Holinski B, Everaert D, Mushahwar V, Stein R. Real-time control of walking using recordings from dorsal root ganglia. J Neural Eng. 2013;10(5):056008.
Google Scholar
Mushahwar V, Aoyagi Y, Stein R, Prochazka A. Movements generated by intraspinal microstimulation in the intermediate gray matter of the anesthetized, decerebrate, and spinal cat. Can J Physiol Pharmacol. 2004;82(8–9):702–14.
Google Scholar
Continue Reading
-

Amar Kanwar Set For Major Retrospective At London’s Serpentine Gallery
Indian filmmaker and artist Amar Kanwar will be the subject of a major retrospective at the Serpentine Gallery in London.
The exhibition will run from September 2026 to January 2027 at Serpentine North. To coincide with the exhibition,…
Continue Reading
-

Atos successfully deployed its advanced sport technologies for the World Para Swimming and World Para Athletics Championships in Asia
Paris, France, October 22, 2025
Atos, a global leader of AI-powered digital transformation, announces today it has successfully delivered the essential services for two landmark Para Sports events taking place almost…
Continue Reading
-
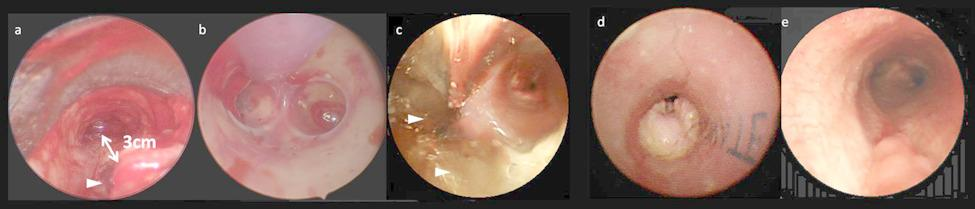
Relapsing polychondritis with catastrophic tracheal injury: extracorporeal membrane oxygenation and silicone Y-stenting for salvage therapy | Journal of Cardiothoracic Surgery
A 29-year-old female diagnosed with RP presented to our emergency department with fever and dyspnea as her primary symptoms. She had a cuffless tracheostomy tube for 12 years due to SGS and TBM. Under the impression of pneumonia with impending…
Continue Reading
-

Lawmakers grill minister over entertainers evading military service
Taipei, Oct. 22 (CNA) Lawmakers pressed Interior Minister Liu Shyh-fang (劉世芳) on Wednesday over a widening military service evasion case involving up to 120 people including actors and vocalists.
Liu told a meeting of the Legislative Yuan’s…
Continue Reading