Goldman Sachs is warning that the risk of a stock-market pullback is rising as investors’ once-strong appetite for risk begins to fade. The bank’s strategists said their Risk Appetite Indicator has fallen to a more neutral level of about 0.2, marking a clear deterioration from the “Goldilocks” conditions that supported markets through the summer. While Goldman’s economists expect U.S. growth to reaccelerate in 2026, the Wall Street investment bank said the probability of a market sell-off outweighs the odds of a large rally. .SPX YTD mountain S & P 500 year to date “The pick-up in drawdown risk has been driven by elevated equity valuations and a weak U.S. business cycle,” the strategists wrote. “Our equity asymmetry framework suggests that the probability of a sell-off is higher than that of a large rally.” The S & P 500 has rebounded aggressively from its April lows to score consecutive record highs, bringing its 2025 gains to more almost 16%. On Friday, cool September inflation data raised investor optimism that the Federal Reserve will keep cutting interest rates, lifting the present value of future profits and possibly boosting corporate earnings too. Goldman is recommending adding downside hedges such as S & P 500 options overlays with drawdown probabilities at current levels. Even so, the bank said it remains “modestly pro-risk” in its overall asset allocation. ( Learn the best 2026 strategies from inside the NYSE with Josh Brown and others at CNBC PRO Live. Tickets and info here . )
Blog
-

Staring at the Black Screen of Death on your Windows PC? 6 steps to fix it
Cesar Cadenas/ZDNET Follow ZDNET: Add us as a preferred source on Google.
ZDNET’s key takeaways
- The Black Screen of Death is incredibly annoying, but often easily diagnosed.
- The most common culprits are outdated software or faulty…
Continue Reading
-

PHERFLOT/IKF-053 Trial at ESMO 2025: Pembrolizumab and Trastuzumab Combined with FLOT in Localized HER2-Positive Esophagogastric Adenocarcinoma
At the ESMO Congress 2025 in Berlin, Dr. Joseph Tintelnot presented the interim analysis of the phase II PHERFLOT/IKF-053 trial (AIO STO 0321), an open-label, multicenter study evaluating whether adding pembrolizumab (anti-PD-1) and trastuzumab (anti-HER2) to perioperative FLOT chemotherapy (5-FU, leucovorin, oxaliplatin, docetaxel) could improve outcomes for patients with HER2-positive localized esophagogastric adenocarcinoma (EGA). Conducted by the AIO Study Group in Germany, this investigation explores the integration of immune- and targeted-therapy principles into a curative-intent treatment paradigm.
Background
For advanced or metastatic HER2-positive EGA, the combination of fluoropyrimidine-platinum chemotherapy with pembrolizumab and trastuzumab is already a standard of care, based on the positive results of the KEYNOTE-811 study. In localized disease, however, perioperative FLOT remains the preferred backbone due to its proven survival advantage over older ECF-based regimens. The PHERFLOT trial was designed to determine whether adding dual HER2 and PD-1 blockade could deepen pathological responses and potentially improve long-term survival without compromising surgical feasibility.
Study Design
PHERFLOT (NCT05504720) is a randomized, open-label, exploratory phase II study enrolling patients with resectable, HER2-positive localized EGA. The co-primary endpoints are 2-year disease-free survival and pathological complete response (pCR). Secondary objectives include safety, progression-free survival, objective response rate, and translational biomarker analyses, such as HER2 expression and PD-L1 combined positive score (CPS).
Patients received standard FLOT chemotherapy combined with pembrolizumab and trastuzumab in both the neoadjuvant and adjuvant settings, followed by curative-intent resection. The current report summarizes baseline characteristics, pathological outcomes, safety, and preliminary translational findings.
Results
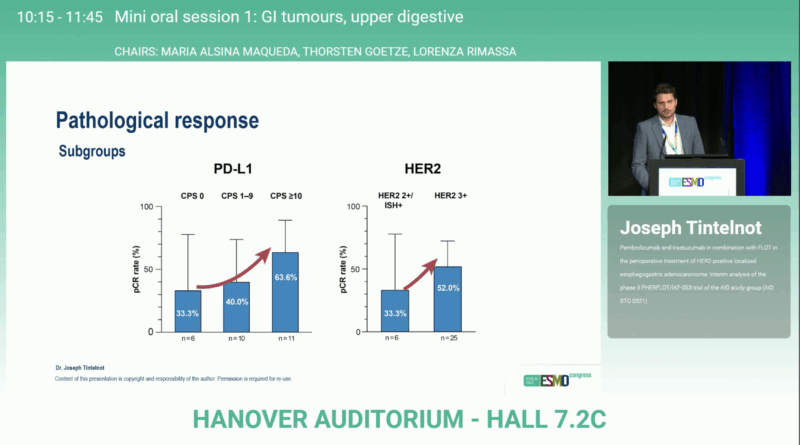
Between March 2023 and May 2024, 31 patients were enrolled across multiple German centers. The median age was 65 years, and most had an ECOG performance status of 0 or 1. The majority of tumors arose at the gastroesophageal junction (77%), while a smaller proportion involved the stomach proper. HER2 overexpression was confirmed by immunohistochemistry, with 80.6% scoring 3+. PD-L1 CPS was ≥1 in 85% and ≥10 in half of evaluated cases, reflecting a generally inflamed tumor phenotype.
Thirty patients proceeded to R0 resection, and 70% experienced no postoperative complications, underscoring the feasibility of combining immune and targeted agents with intensive triplet chemotherapy. The pCR rate reached 48.4% (15 of 31), meeting the co-primary endpoint and far exceeding historical FLOT-alone benchmarks of roughly 15–20%. When subtotal responses were included, two-thirds of patients (67.8%) achieved major regression.
Twenty-one of 31 patients continued beyond perioperative therapy, corresponding to a feasibility rate of 67.7%.
The safety profile was consistent with expectations, except for an increased incidence of Grade 3 diarrhea (38.7%) and a higher re-operation rate (26.7% vs. 10% reported in FLOT4). The most frequent treatment-related events included diarrhea, weight loss, and hematologic toxicities such as neutropenia and anemia. The median hospitalization time was 14 days (vs 15 days in FLOT4), and no 30-day postoperative mortality occurred, confirming the acceptable perioperative safety of the regimen.
Translational Findings
Initial biomarker analyses suggest that PD-L1 expression may correlate with the depth of response, though patient numbers remain small. Ongoing translational work will explore the interaction between HER2 signaling, immune activation, and tumor regression, including immune-cell infiltration and circulating tumor DNA (ctDNA) kinetics. These data may ultimately clarify which subgroups derive the greatest benefit from the triplet combination.
Notably, higher pathological complete response rates were observed in patients with PD-L1 CPS ≥10 (63.6%) and in those with HER2 3+ expression (52%), indicating enhanced treatment sensitivity in these biomarker-enriched subgroups.
You can read the full abstract here.
Conclusions
The interim findings from PHERFLOT/IKF-053 demonstrate that combining pembrolizumab and trastuzumab with perioperative FLOT is feasible, tolerable, and highly active in localized HER2-positive esophagogastric adenocarcinoma. With nearly half of patients achieving complete pathological response and two-thirds achieving major regression, the regimen shows clear biological synergy and potential superiority over current standards.
The long-term results—particularly the 2-year disease-free survival data—will determine whether this intensified perioperative strategy can establish a new benchmark for this molecularly defined subgroup. If confirmed, PHERFLOT could represent a major step toward incorporating immunotherapy and HER2-targeted treatment into curative pathways for upper-GI cancers.

You Can Also Read MATTERHORN Trial at ESMO 2025: Durvalumab Plus FLOT in Resectable Gastric and GEJ Adenocarcinoma by OncoDaily
Continue Reading
-
For some IT pros, the Windows 11 update isn’t an easy switch – IT Brew
- For some IT pros, the Windows 11 update isn’t an easy switch IT Brew
- 4 Windows 11 features I want Microsoft to get rid of right away Pocket-lint
- I ditched Linux for Windows 11 for one week – and found 9 big problems ZDNET
- I’ve Used Windows 11…
Continue Reading
-
U.S. natural gas sector deals surge in 2025 on AI, LNG demand from Asia – Reuters
- U.S. natural gas sector deals surge in 2025 on AI, LNG demand from Asia Reuters
- Natural Gas Stocks Surge: Energy Sector in Focus TipRanks
- Natural Gas: How sellers booked profits amid imminent resurgence FXStreet
- Energy Crunch Ahead: 3 Natural Gas Stocks Set to Gain MarketBeat
- 3 Natural Gas Stocks to Gain From Rising Clean Energy Demand Zacks Investment Research
Continue Reading
-

The Tourist will debut at the San Francisco Short Film Festival
In the realm of independent cinema, few narratives resonate as deeply as the journey of self-discovery. Peter Zerzan’s film, The Tourist, is one such piece of fiction that encapsulates the reality, discomfort and liberation that exist in…
Continue Reading
-

Microsoft Enhances AI Web Browsing With Copilot Mode in Edge Launch
Software giant Microsoft launched new capabilities for its AI-powered web browsing experience, Copilot Mode in Edge. The tool is designed to provide a dynamic, intelligent companion that anticipates, assists and accelerates…
Continue Reading
-

How TARmageddon Compromises Rust Security: A Developer’s Guide
Edera, the security company focused on hardened container runtime security for Kubernetes and AI workloads, has uncovered a new, nasty Rust vulnerability.
Dubbed TARmageddon (CVE-2025-62518), this is a critical flaw in the tokio-tar…
Continue Reading
-
Sinner powers past Bublik, reaches Vienna SFs – ATP Tour
- Sinner powers past Bublik, reaches Vienna SFs ATP Tour
- How could Vienna Open impact Jannik Sinner’s year-end No 1 hopes? Tennis365
- Erste Bank Open Betting Odds and Match Previews for October 24, 2025, Men’s Singles Sportsbook Wire
- Where to watch…
Continue Reading

