Oct. 17 (UPI) — NASA’s Orion spacecraft was moved seven miles to the Vehicle Assembly Building at Kennedy Space Center Thursday, its second-to-last move before its scheduled launch in 2026.
NASA got an exception to allow it to continue work…

Oct. 17 (UPI) — NASA’s Orion spacecraft was moved seven miles to the Vehicle Assembly Building at Kennedy Space Center Thursday, its second-to-last move before its scheduled launch in 2026.
NASA got an exception to allow it to continue work…

The steam rises from the dark underbelly of Seattle’s streets, where neon lights flicker like the last breaths of a dying world. In these streets lies a hidden society, deeply rooted in tradition, politics, and an ever-shifting…

By Charles Passy
The Swedish-born actor, who played the boxer Ivan Drago in ‘Rocky IV,’ has now gone into the vodka-making business and taken a more hands-on approach with his finances
Dolph Lundgren has appeared in more than 75…

Accolades | October 17, 2025
World Tax
World Tax, the International Tax Review’s annual guide to the world’s leading tax advisory practices, has recognized Gibson Dunn in eight categories in the 2026 editions of its EMEA and APAC guides. The firm was recognized in France – General Corporate Tax; France – Tax Controversy; France – Transactional Tax; Hong Kong – General Corporate Tax; Hong Kong – Private Client; UK – General Corporate Tax; UK – Indirect Tax; and UK – Transactional Tax. Partners Sandy Bhogal, Elaine Chen, Jérôme Delaurière, Ben Fryer, Brian Gilchrist, Sanford Stark, and Jeff Trinklein were also recognized individually in the 2026 edition.
The guides were published on October 16, 2026.

We wrap up the show – but not our coverage – with builds from Meerglas…
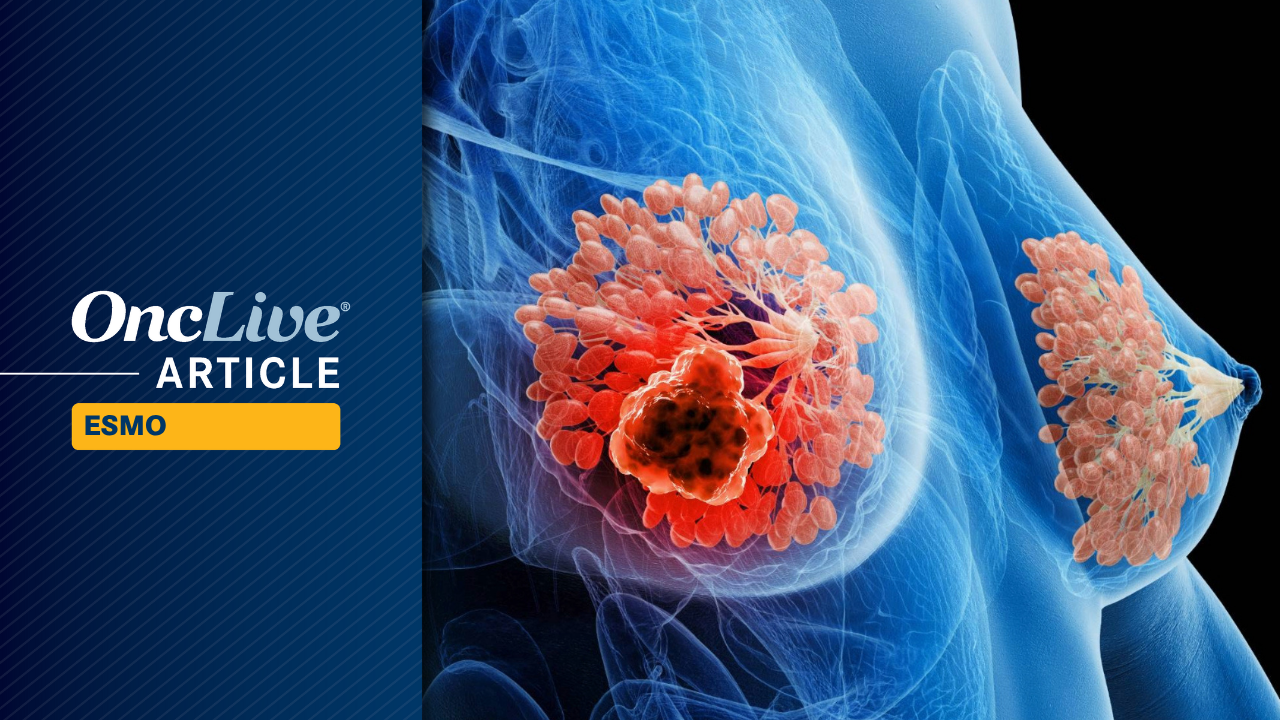
Adjuvant ribociclib (Kisqali) in combination with a nonsteroidal aromatase inhibitor (AI) displayed a durable benefit in terms of invasive disease–free survival (iDFS) compared with an AI alone (n = 2552) in patients with hormone receptor–positive, HER2-negative early breast cancer (HR, 0.716; 95% CI, 0.618-0.829; 1-sided P < .0001), according to data from the 5-year prespecified analysis of the phase 3 NATALEE trial (NCT03701334) presented during the
The median follow-up was 55.4 months. The 36-month iDFS rates in the ribociclib (n = 2594) and AI-alone (n = 2552) arms were 90.8% and 88.0%, respectively; the 60-month iDFS rates were 85.5% and 81.0%, respectively.
Sidebar: Phase 3 NATALEE Trial 5-Year Outcomes: Key Takeaways
“Ribociclib plus an AI continues to reduce the risk of recurrence beyond a 3-year treatment window, supporting its use as adjuvant therapy in a broad population of patients with hormone receptor–positive, HER2-negative early breast cancer at high risk of recurrence,” John Crown, MD, a professor and consultant medical oncologist at St. Vincent’s University Hospital in Dublin, Ireland, said during the presentation.
Notably,
NATALEE was an open-label, multicenter, randomized trial that enrolled adult patients with stage II and III hormone receptor–positive, HER2-negative early breast cancer.1 Patients were permitted to have received prior endocrine therapy up to 12 months before enrollment.
Patients with anatomical stage IIA disease needed to have N0 disease that was grade 2 and had evidence of high risk, defined by a Ki-67 score of at least 20%, an Oncotype DX Breast Recurrence Score of at least 26 or high-risk status via genomic risk profiling, and grade 3 disease; patients with N1 disease in this anatomical stage were also eligible. Patients with anatomical stage IIB disease needed to have N0 or N1 disease. Patients with anatomical stage III disease could have N0 through N3 disease.
Eligible patients were randomly assigned 1:1 to receive ribociclib at 400 mg per day via a 3-weeks-on, 1-week-off dosing schedule for 3 years in combination with a nonsteroidal AI for at least 5 years, or a nonsteroidal AI alone.
The primary end point was iDFS using Standardized Definitions for Efficacy End Points criteria. Secondary end points included relapse-free survival, distant disease–free survival (DDFS), overall survival, patient-reported outcomes, safety and tolerability, and pharmacokinetic measures. Distant recurrence–free survival, as well as gene expression and alterations in circulating tumor (ct) DNA/ctRNA samples, were also evaluated as exploratory end points.
At the May 28, 2025, data cutoff, 62.8% of patients in the combination arm had completed 3 years of treatment with ribociclib, and 36.5% of patients had completed 5 years of AI therapy. AI treatment was ongoing in 27.1% of patients, and 51.4% of patients remained in the follow-up phase.
In the AI-alone arm, 34.4% of patients had completed 5 years of treatment. Therapy was ongoing in 23.7% of patients, and 50.3% of patients were in the follow-up phase.
At the time of this analysis, all patients had stopped receiving therapy for a median of 2 years. No new safety signals were identified with ribociclib, including no delayed toxicities or cumulative effects following therapy. Since the previous 4-year exploratory analysis, with an additional follow-up of 12.9 months, 3 patients had died in the combination arm due to adverse effects (AEs), and 2 others had died in the control arm due to AEs. Three percent of patients had developed secondary primary malignancies in the control arm compared with 2.7% of those in the combination arm.
At a median follow-up of 56.5 months, the 5-year OS rates in the investigational and control arms were 94.1% vs 92.5%, respectively (HR, 0.800; 95% CI, 0.637-1.003; 1-sided P = .026). At a median follow-up of 55.5 months, a significant benefit in terms of DDFS was reported in favor of the ribociclib arm (HR, 0.709; 95% CI, 0.608-0.827). A similar benefit in terms of DRFS was also observed in favor of the investigational arm (HR, 0.699; 95% CI, 0.594-0.824).
Patients experienced an iDFS benefit with the addition of ribociclib to an AI vs an AI alone, irrespective of having N0 (ribociclib arm, n = 285; control arm, n = 329; HR, 0.606; 95% CI, 0.372-0.986) or N-positive (ribociclib arm, n = 2261; control arm, n = 2218; HR, 0.737; 95% CI, 0.631-0.860) nodal status. Notably, an iDFS benefit with ribociclib was reported across all prespecified patient subgroups.
“For the first time, in this 5-year analysis, a clinically meaningful risk reduction of approximately 30% was seen for DDFS or death and DRFS or death. Ribociclib plus an AI showed a continued numerical trend for improved OS,” Crown emphasized in his conclusion.
Disclosures: Crown reported receiving personal fees from Pierre Fabre, Immunocore, Novartis, AstraZeneca, and Regeneron; owning stock with Oncoassure and Akkure; and receiving travel support to meetings from Novartis, MSD Oncology, Pfizer, Roche, AstraZeneca, and Regeneron.

Approval Broadens Indication for TEZSPIRE to a Second Disease Characterized by Epithelial-Driven Inflammation
CRSwNP affects up to approximately 320 million people worldwide and is a complex epithelial-driven inflammatory condition characterized by persistent inflammation and benign polyp growths within the nasal cavity.1-5 People living with CRSwNP commonly experience airflow obstruction and symptoms including congestion and an impaired sense of smell.1-5 For many patients, current therapies such as systemic and intranasal corticosteroids and repeated sinus surgeries do not offer lasting relief.3
“For people living with CRSwNP, every breath can feel like a struggle, and many endure years of recurring symptoms and surgeries without significant relief. The approval of TEZSPIRE represents a meaningful advance, derived from our longstanding focus on complex inflammatory diseases rooted in epithelial biology,” said
The approval by the
“Over 320 million lives globally are disrupted by chronic rhinosinusitis with nasal polyps. The FDA approval of TEZSPIRE brings forward a new treatment option that has demonstrated rapid and sustained symptom improvement, nearly eliminating the need for future surgeries and significantly reducing systemic steroid use,” said Dr.
“Chronic rhinosinusitis with nasal polyps is a persistent and often-overlooked disease that can significantly impact daily life, robbing patients of their ability to breathe without congestion and full sense of smell,” said Kenneth Mendez, President and CEO of the
The safety and tolerability profile of TEZSPIRE in the WAYPOINT trial was generally consistent with its established profile in severe asthma.6 The most frequently reported adverse events in the trial were COVID-19, nasopharyngitis and upper respiratory tract infection.6
Regulatory applications are currently under review in
TEZSPIRE® (tezepelumab-ekko)
TEZSPIRE is indicated for:
TEZSPIRE® (tezepelumab-ekko) Important Safety Information
CONTRAINDICATIONS
Known hypersensitivity to tezepelumab-ekko or excipients.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Hypersensitivity reactions were observed in the clinical trials (e.g., rash and allergic conjunctivitis) following the administration of TEZSPIRE. Postmarketing cases of anaphylaxis have been reported. These reactions can occur within hours of administration, but in some instances have a delayed onset (i.e., days). In the event of a hypersensitivity reaction, consider the benefits and risks for the individual patient to determine whether to continue or discontinue treatment with TEZSPIRE.
Acute Asthma Symptoms or Deteriorating Disease
TEZSPIRE should not be used to treat acute asthma symptoms, acute exacerbations, acute bronchospasm, or status asthmaticus.
Abrupt Reduction of Corticosteroid Dosage
Do not discontinue systemic or inhaled corticosteroids abruptly upon initiation of therapy with TEZSPIRE. Reductions in corticosteroid dose, if appropriate, should be gradual and performed under the direct supervision of a physician. Reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy.
Parasitic (Helminth) Infection
It is unknown if TEZSPIRE will influence a patient’s response against helminth infections. Treat patients with pre-existing helminth infections before initiating therapy with TEZSPIRE. If patients become infected while receiving TEZSPIRE and do not respond to anti-helminth treatment, discontinue TEZSPIRE until infection resolves.
Live Attenuated Vaccines
The concomitant use of TEZSPIRE and live attenuated vaccines has not been evaluated. The use of live attenuated vaccines should be avoided in patients receiving TEZSPIRE.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥ 3%) are:
USE IN SPECIFIC POPULATIONS
There are no available data on TEZSPIRE use in pregnant women to evaluate for any drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Placental transfer of monoclonal antibodies such as tezepelumab-ekko is greater during the third trimester of pregnancy; therefore, potential effects on a fetus are likely to be greater during the third trimester of pregnancy.
Please see the full
Prescribing Information
including Patient Information and Instructions for Use.
You may report side effects related to AstraZeneca products by clicking
here
.
About TEZSPIRE® (tezepelumab-ekko)
TEZSPIRE is a first-in-class human monoclonal antibody that works on the primary source of inflammation: the airway epithelium, which is the first point of contact for viruses, allergens, pollutants and other environmental triggers and insults. Specifically, TEZSPIRE targets and blocks TSLP, a key epithelial cytokine that sits at the top of multiple inflammatory cascades and initiates an overreactive immune response to allergic, eosinophilic and other types of airway inflammation associated with severe asthma.8, 9 TSLP is released in response to multiple triggers associated with asthma exacerbations, including allergens, viruses and other airborne particles.9,10
Expression of TSLP is increased in the airways of patients with asthma and has been correlated with disease severity.5,8 Blocking TSLP may prevent the release of pro-inflammatory cytokines by immune cells, resulting in the prevention of asthma exacerbations and improved asthma control.8,9,11
TEZSPIRE is currently approved as a pre-filled, single-use pen and auto-injector for self-administration for the treatment of severe asthma in the U.S., Europe, Japan, and more than 60 countries across the globe,12-15 and for the treatment of chronic rhinosinusitis with nasal polyps in the U.S.
Beyond severe asthma and CRSwNP, TEZSPIRE is also in development for other potential indications including chronic obstructive pulmonary disease (COPD) and eosinophilic esophagitis (EoE).16,17 In October 2021, tezepelumab was granted Orphan Drug Designation by the FDA for the treatment of EoE.
About Chronic Rhinosinusitis with Nasal Polyps (CRSwNP [nasal polyps])
CRSwNP is a complex inflammatory disorder characterized by persistent inflammation of the nasal mucosa accompanied by benign growths, called nasal polyps.2,3 Nasal polyps can block nasal passages and lead to breathing problems, difficulty in sense of smell, nasal discharge, and other adverse effects on quality of life.1,4,5
Epithelial dysfunction and inflammation are important characteristics of chronic rhinosinusitis and impede the ability of the epithelium to act as a physical and immunological barrier against the external environment.18 Estimates suggest that up to 56% of patients with CRSwNP have comorbid asthma. Thymic stromal lymphopoietin (TSLP) is an epithelial cytokine that has been implicated in shared pathophysiological processes underlying severe asthma and CRSwNP.19,20
Current treatments for CRSwNP include intranasal and/or systemic corticosteroids, surgery and biologic medication.3,5,21-26
About the Phase 3 WAYPOINT Trial
WAYPOINT was a double-blind, multi-center, randomized, placebo-controlled, parallel group trial designed to evaluate the efficacy and safety of tezepelumab in adults with uncontrolled CRSwNP.6,7,27 Participants received tezepelumab or placebo, administered via subcutaneous injection. The trial also included a post-treatment follow-up period of 12-24 weeks for participants who completed the 52-week treatment period.6,7,27
The co-primary endpoints of the trial, were change from baseline in total nasal polyp size, measured by the endoscopic total Nasal Polyp Score, and change from baseline in bi-weekly mean nasal congestion, measured by the participant reported Nasal Congestion Score evaluated as part of the daily Nasal Polyposis Symptom Diary.6,27 Key secondary endpoints included loss of smell; improvement in disease specific health-related quality of life as measured by SinoNasal Outcome Test (SNOT-22) score;
About the Amgen and AstraZeneca Collaboration
In 2020, Amgen and AstraZeneca updated the 2012 collaboration agreement for TEZSPIRE. Both companies will continue to share costs and profits equally after payment by AstraZeneca of a mid-single-digit royalty to Amgen. AstraZeneca continues to lead development and Amgen continues to lead manufacturing. All aspects of the collaboration are under the oversight of joint governing bodies. Under the amended agreement, in North America, Amgen, as the principal, recognizes product sales of TEZSPIRE in the
About Amgen
In 2024,
For more information, visit Amgen.com and follow
This news release contains forward-looking statements that are based on the current expectations and beliefs of
No forward-looking statement can be guaranteed and actual results may differ materially from those we project. Discovery or identification of new product candidates or development of new indications for existing products cannot be guaranteed and movement from concept to product is uncertain; consequently, there can be no guarantee that any particular product candidate or development of a new indication for an existing product will be successful and become a commercial product. Further, preclinical results do not guarantee safe and effective performance of product candidates in humans. The complexity of the human body cannot be perfectly, or sometimes, even adequately modeled by computer or cell culture systems or animal models. The length of time that it takes for us to complete clinical trials and obtain regulatory approval for product marketing has in the past varied and we expect similar variability in the future. Even when clinical trials are successful, regulatory authorities may question the sufficiency for approval of the trial endpoints we have selected. We develop product candidates internally and through licensing collaborations, partnerships and joint ventures. Product candidates that are derived from relationships may be subject to disputes between the parties or may prove to be not as effective or as safe as we may have believed at the time of entering into such relationship. Also, we or others could identify safety, side effects or manufacturing problems with our products, including our devices, after they are on the market.
Our results may be affected by our ability to successfully market both new and existing products domestically and internationally, clinical and regulatory developments involving current and future products, sales growth of recently launched products, competition from other products including biosimilars, difficulties or delays in manufacturing our products and global economic conditions, including those resulting from geopolitical relations and government actions. In addition, sales of our products are affected by pricing pressure, political and public scrutiny and reimbursement policies imposed by third-party payers, including governments, private insurance plans and managed care providers and may be affected by regulatory, clinical and guideline developments and domestic and international trends toward managed care and healthcare cost containment. Furthermore, our research, testing, pricing, marketing and other operations are subject to extensive regulation by domestic and foreign government regulatory authorities. Our business may be impacted by government investigations, litigation and product liability claims. In addition, our business may be impacted by the adoption of new tax legislation or exposure to additional tax liabilities. Further, while we routinely obtain patents for our products and technology, the protection offered by our patents and patent applications may be challenged, invalidated or circumvented by our competitors, or we may fail to prevail in present and future intellectual property litigation. We perform a substantial amount of our commercial manufacturing activities at a few key facilities, including in
CONTACT:
REFERENCES
View original content to download multimedia:https://www.prnewswire.com/news-releases/fda-approves-tezspire-for-chronic-rhinosinusitis-with-nasal-polyps-302587969.html
SOURCE

British actor Samantha Eggar, the Oscar-nominated star of films including “The Collector,” “Doctor Dolittle” and David Cronenberg’s “The Brood,” has died. She was 86.
Eggar died Wednesday evening, her daughter Jenna Stern announced…
