The Federal Public Service Commission (FPSC) has announced details of vacancies reserved under the Central Superior Services (CSS) Examination 2024, revealing a total of 364 positions across federal departments and organisations.
According to an…

The Federal Public Service Commission (FPSC) has announced details of vacancies reserved under the Central Superior Services (CSS) Examination 2024, revealing a total of 364 positions across federal departments and organisations.
According to an…

Good afternoon! This week’s Patch Notes is coming in HOT and for that I apologise. It’s been a hectic few days.
I’m preparing to head to Bangkok for Gamescom Asia on Tuesday and juggling some rather last-minute admin (yes, that includes securing…

 Trustees of the British Museum
Trustees of the British MuseumThe bronze figurehead of a goddess is one of the highlights of a new exhibition featuring Sudan’s ancient Kingdom of Kush, going on display…
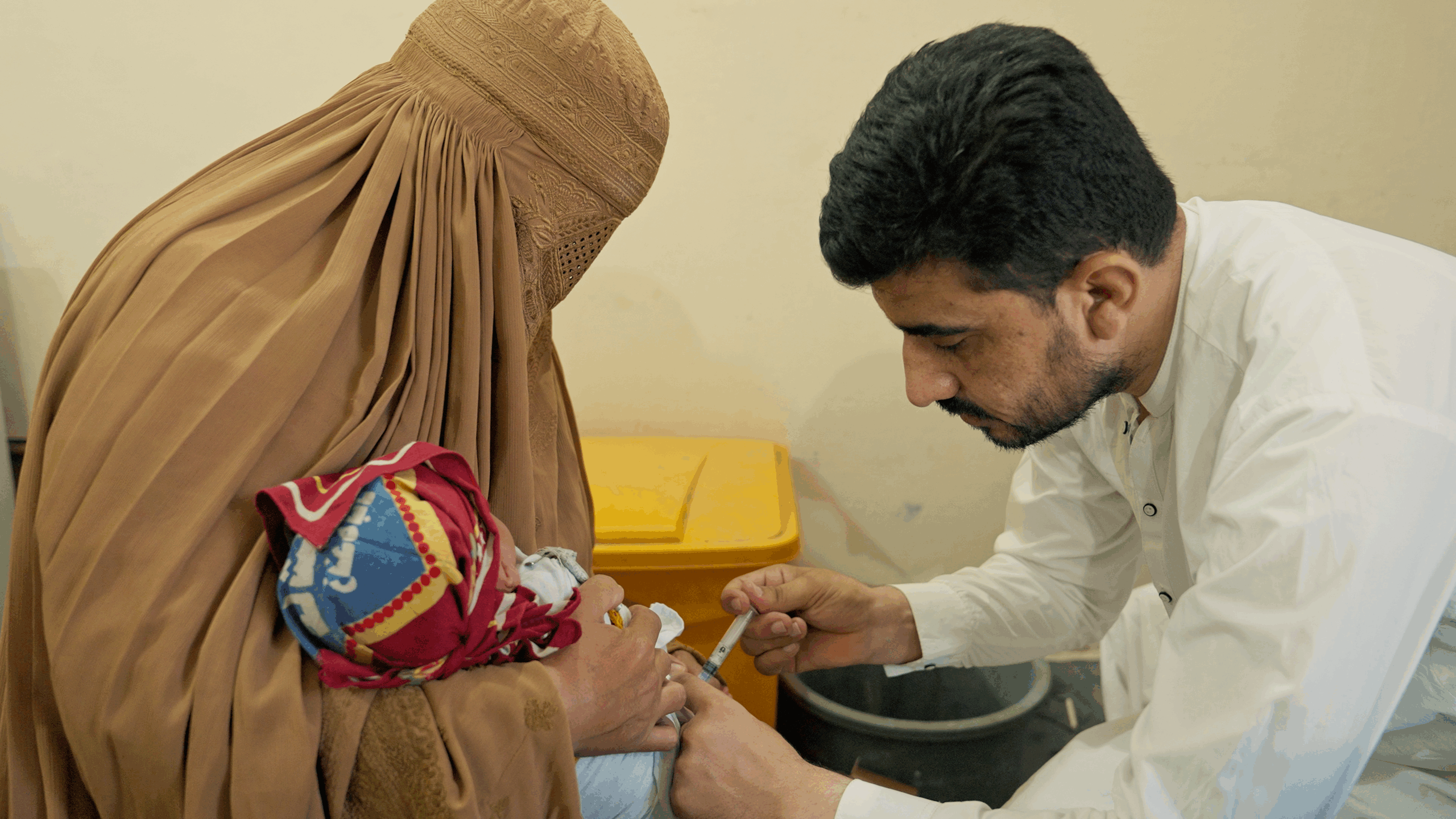
“I couldn’t walk because of polio, so my brother used to carry my bag to school every day. When other…

Bombardier announced today its participation in the 2025 edition of the NBAA Business Aviation Convention & Exhibition (NBAA-BACE), taking place October 14–16 at Henderson Executive Airport in Las Vegas. As a global leader in business aviation, Bombardier will showcase three of its most iconic aircraft on static display – the Global 7500, Global 6500, and Challenger 3500 – and will also highlight its top-ranked, comprehensive service offering on site.
“Our aircraft are designed to inspire, and they stand out as some of the most compelling examples of innovation and craftsmanship at this year’s show, complemented by our extensive service offering that ensures an exceptional ownership experience,” said Éric Martel, President and Chief Executive Officer, Bombardier. “The Bombardier Global 7500, Global 6500, and Challenger 3500 each bring something exceptional to the table, and with the Global 8000 on the horizon as the fastest business jet in the world, we continue to redefine what’s possible in business aviation.”
Visitors to the static display will have the opportunity to explore the spacious and refined interiors of each jet—including the record-setting Bombardier Global 7500, renowned for its industry-leading speed and landing capabilities; the Global 6500, offering outstanding versatility and long-range performance; and the Challenger 3500, which delivers impressive speed and efficiency. All three aircraft are engineered to provide Bombardier’s signature smooth ride, ensuring exceptional comfort on every mission.
Media representatives are invited to attend two Bombardier events at NBAA-BACE in Las Vegas for exclusive access to the company’s latest announcements. The first media event will be held on Monday, October 13 at 11:00 a.m. (Pacific Time). A second event—a special unveiling celebration—will take place at Bombardier’s static display at Henderson Executive Airport on Tuesday, October 14 at 2:00 p.m. (Pacific Time).
For media inquiries or to schedule aircraft tours, please contact Bombardier’s Public Relations team.
At Bombardier (BBD-B.TO), we design, build, modify and maintain the world’s best-performing aircraft for the world’s most discerning people and businesses, governments and militaries. That means not simply exceeding standards, but understanding customers well enough to anticipate their unspoken needs.
For them, we are committed to pioneering the future of aviation—innovating to make flying more reliable, efficient and sustainable. And we are passionate about delivering unrivaled craftsmanship and care, giving our customers greater confidence and the elevated experience they deserve and expect. Because people who shape the world will always need the most productive and responsible ways to move through it.
Bombardier customers operate a fleet of more than 5,100 aircraft, supported by a vast network of Bombardier team members worldwide and 10 service facilities across six countries. Bombardier’s performance-leading jets are proudly manufactured in aerostructure, assembly and completion facilities in Canada, the United States and Mexico. In 2024, Bombardier was honoured with the prestigious “Red Dot: Best of the Best” award for Brands and Communication Design.
For corporate news and information, including Bombardier’s Sustainability report, as well as the company’s initiative to cover all its flight operations with a Sustainable Aviation Fuel (SAF) blend utilizing the Book-and-Claim system visit
bombardier.com.
Learn more about Bombardier’s industry-leading products and customer service network at bombardier.com. Follow us on X @Bombardier.
General media contact webform
Christina Lemyre McCraw
+1-514-497-4928
christina.lemyremccraw@aero.bombardier.com
Bombardier, Global, Global 8000, Global 7500, Global 6500 and Challenger 3500 are registered or unregistered trademarks of Bombardier Inc. or its subsidiaries.
TITUSVILLE, N.J. (October 10, 2025) — Johnson & Johnson (NYSE: JNJ) announced today that 17 abstracts featuring new clinical and real-world data will be presented at the annual European College of Neuropsychopharmacology (ECNP) Congress, taking place October 11-14 in Amsterdam, The Netherlands. Presentations include the latest research from across the Company’s neuropsychiatry portfolio, including major depressive disorder (MDD), treatment-resistant depression (TRD), and schizophrenia.
“MDD is a complex disorder that can manifest in different ways for each individual, and the traditional one-size-fits-all treatment approach often results in mixed patient outcomes,” said Bill Martin, Ph.D., Global Neuroscience Therapeutic Area Head, Johnson & Johnson Innovative Medicine.1,2 “At Johnson & Johnson, we are committed to advancing innovative and differentiated therapies through a targeted and patient-first approach, and our data at ECNP strongly reflects our relentless focus on this commitment.”
Key presentations include:
Johnson & Johnson will present the following posters at ECNP Congress on October 12 from 8:00 – 8:30 a.m. CET (e-posters), October 13 from 12:35 – 2:00 p.m. CET, and October 14 from 12:35 – 2:00 p.m. CET.
| Poster # | Title |
| Major Depressive Disorder | |
| EP03-0245 | Adjunctive Lumateperone 42 mg Treatment in Major Depressive Disorder: Efficacy in Anhedonia and Across Broad Range of Depressive Symptoms |
| PS03-2109 | Efficacy of Adjunctive Lumateperone 42 mg Treatment Across Depression and Anhedonia Symptoms in Major Depressive Disorder |
| PS04-3102 | Evaluation of Sexual Function With Adjunctive Lumateperone in Patients With Major Depressive Disorder |
| EP03-0243 | Long-Term Adjunctive Lumateperone Treatment in Major Depressive Disorder: Results From a Six-Month Open-Label Extension Study |
| PS03-2108 | Beyond Inflammation: Unveiling Novel Molecular Mechanisms in Major Depressive Disorder and Antidepressant Response in a Cohort Stratified by Inflammatory Status |
| PS02-1123 | Seltorexant: A Safe and Well-Tolerated Adjunctive Treatment for Adolescent Major Depressive Disorder With Comparable Pharmacokinetics to Adults |
| PS03-2143 | Factors Associated With Long-Term Hypnotics Use in Depression |
| Oral Presentation | Developments in Adjunctive Treatment: Seltorexant Versus Quetiapine in Managing Major Depressive Disorder With Insomnia Symptoms |
| Treatment-Resistant Depression | |
| PS02-1220 | Efficacy and Safety of 4 Months of Treatment With Esketamine Nasal Spray Monotherapy in Adult Patients With Treatment-Resistant Depression |
| PS01-0124 | Early Dose Management and Up-Titration of Esketamine in the Double-Blind Induction Phase of the Randomized, Active-Controlled, Phase 3 TRANSFORM-2 Study |
| PS04-3215 | Expert Consensus on Decision-Making Factors for Continuation of Esketamine Nasal Spray in Treatment-Resistant Depression: A Delphi Method |
| PS02-1216 | ECHO: Study Design and Baseline Characteristics of a Non-Interventional Cohort Study of Esketamine Nasal Spray in Treatment-Resistant Depression |
| PS02-1219 | Patient Characteristics Associated With Relative Benefit of Esketamine Nasal Spray Versus Quetiapine Extended Release on Achieving Remission in ESCAPE-TRD Study |
| PS02-1111 | Evolution of Clinical Dimensions and Safety in Patients With Major Depressive Disorder Treated by Esketamine: The French Real-World ELLIPSE Study |
| PS01-0076 | Prevalence, Incidence, and Therapy of (Treatment-Resistant) Depression in Germany: A Sickness Funds Analysis |
| Schizophrenia | |
| PS01-0220 | Impact of Paliperidone Palmitate 1-Month and 3-Month Long-Acting Injectables on Clinical and Psychosocial Outcomes in Rwandan Patients With Schizophrenia |
| PS04-3104 | Lumateperone For The Prevention of Relapse in Patients with Schizophrenia: Results From a Double-Blind, Placebo-Controlled, Randomized Withdrawal, Phase 3 Trial |
ABOUT MAJOR DEPRESSIVE DISORDER (MDD)
MDD is one of the most common psychiatric disorders and a leading cause of disability worldwide, impacting an estimated 332 million people – or about 4 percent of the population.8,9 In 2023, approximately 22 million adults in the U.S. had at least one major depressive episode.10 While depression is typically treated with a “one-size-fits-all” approach, no two cases are the same. MDD is a complex, heterogeneous disorder involving multiple regions of the brain and presenting with as many as 256 unique symptom combinations.1,2 As a result, responses to treatment vary widely. With current standard-of-care oral antidepressants, 2 in 3 people living with MDD continue to experience residual or persistent symptoms.11 Moreover, MDD is a risk factor for the development and worsening of a range of comorbidities, illustrating the importance of integrating mental and general health care.12
Insomnia is one of the most common symptoms of MDD, affecting more than 80 percent of people living with MDD.13 Disturbed sleep and insomnia symptoms have a significant impact on a patient’s quality of life and exacerbate the risk of depressive relapse and suicide.14,15
Approximately one-third of adults with MDD will not respond to oral antidepressants alone and are considered to have treatment-resistant depression (TRD), which is often defined as inadequate response to two or more oral antidepressants that were administered at an adequate dose for an adequate duration.16,17 TRD has a significant negative impact on the lives of those affected and has one of the highest economic burdens of all psychiatric disorders.17 Patients often cycle through multiple oral medications, waiting 4-6 weeks for potential relief.18 Based on the STAR*d study, after trying their third oral antidepressant, approximately 86 percent of patients do not achieve remission.18
ABOUT CAPLYTA®
CAPLYTA® (lumateperone) 42 mg is an oral, once daily atypical antipsychotic approved for the treatment of adults with schizophrenia, as well as depressive episodes associated with bipolar I or II disorder (bipolar depression), as monotherapy, and as adjunctive therapy with lithium or valproate.
While its exact mechanism of action is unknown, CAPLYTA® is characterized by high serotonin 5-HT2A receptor occupancy and lower amounts of dopamine D2 receptor occupancy at therapeutic doses.
A supplemental new drug application (sNDA) for CAPLYTA® as an adjunctive treatment for adults with major depressive disorder is currently under U.S. Food and Drug Administration review.
ABOUT SELTOREXANT
Seltorexant, an investigational first-in-class therapy, is a selective antagonist of the human orexin-2 receptor currently being developed as an adjunctive treatment for adults with MDD with insomnia symptoms. Seltorexant selectively antagonizes the orexin-2 receptors, potentially improving mood symptoms and restoring sleep without next-day sedation in patients with depression.19 When orexin-2 receptors are stimulated for too long or at inappropriate times, their activation can cause hyperarousal manifestations, including insomnia and excessive cortisol release, which may contribute to depression and insomnia.20,21 Seltorexant is the only investigational therapy being studied in MDD that is believed to work by normalizing the overactivation of the orexin-2 receptors, thereby addressing the underlying biology that contributes to depression and causes insomnia symptoms.
ABOUT SPRAVATO®
SPRAVATO® (esketamine) CIII nasal spray is approved by the U.S. Food and Drug Administration alone or in conjunction with an oral antidepressant for adults with MDD when they have inadequate response to at least two oral antidepressants (TRD) and depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior in conjunction with an oral antidepressant. It is a non-selective, non-competitive antagonist of the N-methyl-D-aspartate (NMDA) receptor and is believed to work differently than traditional antidepressants by acting on a pathway in the brain that affects glutamate. The mechanism by which esketamine exerts its antidepressant effect is unknown. To date, SPRAVATO® has been approved in 79 markets and administered to more than 150,000 patients worldwide.
ABOUT SCHIZOPHRENIA
Schizophrenia is a complex, chronic brain disorder that affects how people think, feel, speak, and act. It affects up to an estimated 2.8 million adults in the U.S. yet remains widely misunderstood and insufficiently treated.22 Symptoms vary by person, but confusion and distortions in perceptions, emotions, and behavior are common.23 Evidence shows that the first three to five years after diagnosis – “the critical period” – from symptom onset are key for a patient’s treatment, as this is when the condition progresses most rapidly.24,25 A comprehensive treatment plan, which may include medication, therapy, and psychosocial services, can be critical in delaying the time to relapse for adults with schizophrenia.26
ABOUT JOHNSON & JOHNSON’S SCHIZOPHRENIA PORTFOLIO
Johnson & Johnson’s portfolio of schizophrenia therapies offers the broadest range of oral and long-acting injectable treatment options to support each patient’s individual treatment journey. The Company’s long-acting injectable treatments for adults with schizophrenia provides the most varied range of dosing options and the longest-lasting schizophrenia treatments with each dose available, including INVEGA SUSTENNA® (1-month paliperidone palmitate), INVEGA TRINZA® (3-month paliperidone palmitate), and INVEGA HAFYERA® (6-month paliperidone palmitate), all of which are administered in a clinical setting by a medical professional.23,24
CAPLYTA® is a once-daily oral therapy approved to treat adults with schizophrenia. A supplemental New Drug Application (sNDA) for CAPLYTA® with long-term data evaluating the safety and efficacy of the medication for the prevention of relapse in schizophrenia was recently
submitted to the U.S. Food and Drug Administration.
CAPLYTA® IMPORTANT SAFETY INFORMATION
CAPLYTA® (lumateperone) is indicated in adults for the treatment of schizophrenia and depressive episodes associated with bipolar I or II disorder (bipolar depression) as monotherapy and as adjunctive therapy with lithium or valproate.
Important Safety Information
Boxed Warnings:
· Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. CAPLYTA is not approved for the treatment of patients with dementia-related psychosis.
· Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adults in short-term studies. All antidepressant-treated patients should be closely monitored for clinical worsening, and for emergence of suicidal thoughts and behaviors. The safety and effectiveness of CAPLYTA have not been established in pediatric patients.
Contraindications: CAPLYTA is contraindicated in patients with known hypersensitivity to lumateperone or any components of CAPLYTA. Reactions have included pruritus, rash (e.g., allergic dermatitis, papular rash, and generalized rash), and urticaria.
Warnings & Precautions: Antipsychotic drugs have been reported to cause:
Drug Interactions: CAPLYTA should not be used with CYP3A4 inducers. Dose reduction is recommended for concomitant use with strong CYP3A4 inhibitors or moderate CYP3A4 inhibitors.
Special Populations: Newborn infants exposed to antipsychotic drugs during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. Dose reduction is recommended for patients with moderate or severe hepatic impairment.
Adverse Reactions: The most common adverse reactions in clinical trials with CAPLYTA vs. placebo were somnolence/sedation, dizziness, nausea, and dry mouth.
CAPLYTA is available in 10.5 mg, 21 mg, and 42 mg capsules.
Please click here to see full Prescribing Information including Boxed Warnings.
SPRAVATO® IMPORTANT SAFETY INFORMATION
What is SPRAVATO® (esketamine) CIII nasal spray? SPRAVATO® is a prescription medicine used:
SPRAVATO® is not for use as a medicine to prevent or relieve pain (anesthetic). It is not known if SPRAVATO® is safe or effective as an anesthetic medicine.
It is not known if SPRAVATO® is safe and effective for use in preventing suicide or in reducing suicidal thoughts or actions. SPRAVATO® is not for use in place of hospitalization if your healthcare provider determines that hospitalization is needed, even if improvement is experienced after the first dose of SPRAVATO®.
It is not known if SPRAVATO® is safe and effective in children.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about SPRAVATO®?
SPRAVATO® can cause serious side effects, including:
o Pay close attention to any changes, especially sudden changes, in mood, behavior, thoughts, or feelings, or if you develop suicidal thoughts or actions.
o Tell your healthcare provider right away if you have any new or sudden changes in mood, behavior, thoughts, or feelings, or if you develop suicidal thoughts or actions.
o Keep all follow-up visits with your healthcare provider as scheduled. Call your healthcare provider between visits as needed, especially if you have concerns about symptoms.
Tell your healthcare provider or get emergency help right away if you or your family member have any of the following symptoms, especially if they are new, worse, or worry you:
| · thoughts about suicide or dying · new or worse depression · feeling very agitated or restless · trouble sleeping (insomnia) · acting aggressive, being angry or violent · an extreme increase in activity and talking (mania) |
· suicide attempts · new or worse anxiety · panic attacks · new or worse irritability · acting on dangerous impulses · other unusual changes in behavior or mood |
Do not take SPRAVATO® if you:
If you are not sure if you have any of the above conditions, talk to your healthcare provider before taking SPRAVATO®.
Before you take SPRAVATO®, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines that you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Taking SPRAVATO® with certain medicine may cause side effects.
Especially tell your healthcare provider if you take central nervous system (CNS) depressants, psychostimulants, or monoamine oxidase inhibitors (MAOIs) medicines. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How will I take SPRAVATO®?
What should I avoid while taking SPRAVATO®?
Do not drive, operate machinery, or do anything where you need to be completely alert after taking SPRAVATO®. Do not take part in these activities until the next day following a restful sleep. See “What is the most important information I should know about SPRAVATO®?”
What are the possible side effects of SPRAVATO®?
SPRAVATO® may cause serious side effects including:
See “What is the most important information I should know about SPRAVATO®?”
Increased blood pressure. SPRAVATO® can cause a temporary increase in your blood pressure that may last for about 4 hours after taking a dose. Your healthcare provider will check your blood pressure before taking SPRAVATO® and for at least 2 hours after you take SPRAVATO®. Tell your healthcare provider right away if you get chest pain, shortness of breath, sudden severe headache, change in vision, or seizures after taking SPRAVATO®.
Problems with thinking clearly. Tell your healthcare provider if you have problems thinking or remembering.
Bladder problems. Tell your healthcare provider if you develop trouble urinating, such as a frequent or urgent need to urinate, pain when urinating, or urinating frequently at night.
The most common side effects of SPRAVATO® include:
| · feeling disconnected from yourself, your thoughts, feelings and things around you
· dizziness · nausea · feeling sleepy · spinning sensation · decreased feeling of sensitivity (numbness) |
· feeling anxious
· lack of energy · increased blood pressure · vomiting · feeling drunk · headache · feeling very happy or excited |
If these common side effects occur, they usually happen right after taking SPRAVATO® and go away the same day.
These are not all the possible side effects of SPRAVATO®.
Call your doctor for medical advice about side effects. You may report side effects to Johnson & Johnson at 1-800-526-7736, or to the FDA at 1-800-FDA-1088.
Please see full
Prescribing Information, including Boxed WARNINGS, and
Medication Guide for SPRAVATO® and discuss any questions you may have with your healthcare provider.
cp-170363v4
INVEGA SUSTENNA®, INVEGA TRINZA®, INVEGA HAFYERA® IMPORTANT SAFETY INFORMATION
INDICATIONS
INVEGA HAFYERA® (6-month paliperidone palmitate) is a prescription medicine given by injection every 6 months by a healthcare professional and used to treat schizophrenia. INVEGA HAFYERA® is used in adults who have been treated with either:
INVEGA TRINZA® is a prescription medicine given by injection every 3 months by a healthcare professional and used to treat schizophrenia. INVEGA TRINZA® is used in people who have been adequately treated with INVEGA SUSTENNA® for at least 4 months.
INVEGA SUSTENNA® is a prescription medicine given by injection by a healthcare professional.
INVEGA SUSTENNA® is used to treat schizophrenia in adults.
INVEGA SUSTENNA®, INVEGA TRINZA®, INVEGA HAFYERA® IMPORTANT SAFETY INFORMATION
What is the most important information I should know about INVEGA HAFYERA®, INVEGA TRINZA® and INVEGA SUSTENNA®?
INVEGA HAFYERA®, INVEGA TRINZA® and INVEGA SUSTENNA® may cause serious side effects, including:
Do not receive INVEGA HAFYERA®, INVEGA TRINZA® or INVEGA SUSTENNA® if you are allergic to paliperidone, paliperidone palmitate, risperidone, or any of the ingredients in INVEGA HAFYERA®, INVEGA TRINZA® or INVEGA SUSTENNA®. See the end of the Patient Information leaflet in the full Prescribing Information for a complete list of INVEGA HAFYERA®, INVEGA TRINZA® and INVEGA SUSTENNA® ingredients.
Before you receive INVEGA HAFYERA®, INVEGA TRINZA® or INVEGA SUSTENNA®, tell your healthcare professional about all your medical conditions, including if you:
Tell your healthcare professional about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. INVEGA HAFYERA®, INVEGA TRINZA® and INVEGA SUSTENNA® may affect the way other medicines work, and other medicines may affect how INVEGA HAFYERA®, INVEGA TRINZA® and INVEGA SUSTENNA® works.
Your healthcare provider can tell you if it is safe to receive INVEGA HAFYERA®, INVEGA TRINZA® or INVEGA SUSTENNA® with your other medicines. Do not start or stop any medicines during treatment with INVEGA HAFYERA®, INVEGA TRINZA® or INVEGA SUSTENNA® without talking to your healthcare provider first. Know the medicines you take. Keep a list of them to show to your healthcare professional or pharmacist when you get a new medicine.
Patients (particularly the elderly) taking antipsychotics with certain health conditions or those on long-term therapy should be evaluated by their healthcare professional for the potential risk of falls.
How will I receive INVEGA HAFYERA®, INVEGA TRINZA® or INVEGA SUSTENNA®?
What should I avoid while receiving INVEGA HAFYERA®, INVEGA TRINZA® or INVEGA SUSTENNA®?
INVEGA HAFYERA®, INVEGA TRINZA® and INVEGA SUSTENNA® may cause serious side effects, including:
The most common side effects of INVEGA HAFYERA® include: injection site reactions, weight gain, headache, upper respiratory tract infections, feeling restlessness or difficulty sitting still, slow movements, tremors, stiffness and shuffling walk.
The most common side effects of INVEGA TRINZA® include: injection site reactions, weight gain, headache, upper respiratory tract infections, feeling restlessness or difficulty sitting still, slow movements, tremors, stiffness and shuffling walk.
The most common side effects of INVEGA SUSTENNA® include: injection site reactions; sleepiness or drowsiness; dizziness; feeling of inner restlessness or needing to be constantly moving; abnormal muscle movements, including tremor (shaking), shuffling, uncontrolled involuntary movements, and abnormal movements of your eyes.
Tell your healthcare professional if you have any side effect that bothers you or does not go away. These are not all the possible side effects of INVEGA HAFYERA®, INVEGA TRINZA® or INVEGA SUSTENNA®. For more information, ask your healthcare professional or pharmacist.
Call your healthcare professional for medical advice about side effects. You may report side effects of prescription drugs to the FDA at 1-800-FDA-1088.
General information about the safe and effective use of INVEGA HAFYERA®, INVEGA TRINZA® or INVEGA SUSTENNA®
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet.
Do not use INVEGA HAFYERA®, INVEGA TRINZA® or INVEGA SUSTENNA® for a condition for which it was not prescribed. You can ask your pharmacist or healthcare professional for information about INVEGA HAFYERA®, INVEGA TRINZA® or INVEGA SUSTENNA® that is written for healthcare professionals.
For more information, go to
www.invegahafyera.com,
www.invegatrinza.com or
www.invegasustenna.com or call
1-800-526-7736.
Please click to read the full Prescribing Information, including Boxed WARNING, for
INVEGA HAFYERA®,
INVEGA TRINZA® and
INVEGA SUSTENNA® and discuss any questions you have with your healthcare professional.
cp-256259v4
https://www.intracellulartherapies.com/docs/caplyta_pi.pdf
ABOUT JOHNSON & JOHNSON
At Johnson & Johnson, we believe health is everything. Our strength in healthcare innovation empowers us to build a world where complex diseases are prevented, treated, and cured, where treatments are smarter and less invasive, and solutions are personal. Through our expertise in Innovative Medicine and MedTech, we are uniquely positioned to innovate across the full spectrum of healthcare solutions today to deliver the breakthroughs of tomorrow and profoundly impact health for humanity.
Learn more at
http://www.jnj.com or at
www.innovativemedicine.jnj.com. Follow us at
@JNJInnovMed.
Cautions Concerning Forward-Looking Statements
This press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 related to CAPLYTA®, Seltorexant, SPRAVATO®, INVEGA HAFYERA®, INVEGA TRINZA® and INVEGA SUSTENNA®. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to: challenges and uncertainties inherent in product research and development, including the uncertainty of clinical success and of obtaining regulatory approvals; uncertainty of commercial success; manufacturing difficulties and delays; competition, including technological advances, new products, and patents attained by competitors; challenges to patents; product efficacy or safety concerns resulting in product recalls or regulatory action; changes in behavior and spending patterns of purchasers of healthcare products and services; changes to applicable laws and regulations, including global healthcare reforms; and trends toward healthcare cost containment. A further list and descriptions of these risks, uncertainties, and other factors can be found in Johnson & Johnson’s most recent Annual Report on Form 10-K, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in Johnson & Johnson’s subsequent Quarterly Reports on Form 10-Q and other filings with the U.S. Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com, www.investor.jnj.com or on request from Johnson & Johnson. Johnson & Johnson does not undertake to update any forward-looking statement as a result of new information or future events or developments.
Footnotes:
© Johnson & Johnson 2025. All rights reserved.

NEW YORK — NEW YORK (AP) — Prince Harry and Meghan Markle urged parents to stand against social media companies that they said prey upon children with exploitative algorithms as the “explosion of unregulated artificial intelligence” adds…

MANILA, Philippines — MANILA, Philippines (AP) — An offshore earthquake with a preliminary magnitude of 7.4 hit off a southern Philippine province Friday morning, and a hazardous tsunami was possible nearby.
The Philippine Institute of…

Come what may, Jean Nouvel will always have Paris. The City of Lights has been the stage and stomping ground of French architecture’s vieux terrible since the early 1980s. Yet the building that first made his name – the Institut du Monde…