- Interior minister briefs PM Shehbaz on law and order situation Dawn
- TLP leadership to face music: Naqvi The Express Tribune
- No one will be allowed to spread unrest, warn federal ministers The Nation (Pakistan )
- Naqvi meets Owais Noorani Daily…
Blog
-
Interior minister briefs PM Shehbaz on law and order situation – Dawn
-

Vierge and Lecuona round out the 2025 season…
After a final warm-up, the factory team turned its attentions to the Superpole race. Having qualified eighth, Vierge was straight up into fifth with his CBR1000RR-R. Passing Lowes for fourth on lap two, the home rider was lapping faster than…
Continue Reading
-

Bangladesh garment exporters fear $1bn losses after huge airport fire | Business and Economy News
The fire gutted import cargo terminals areas at Dhaka airport, destroying an estimated $1bn of ‘urgent air shipments’.
Published On 19 Oct 2025
A fire that decimated a cargo complex in…
Continue Reading
-
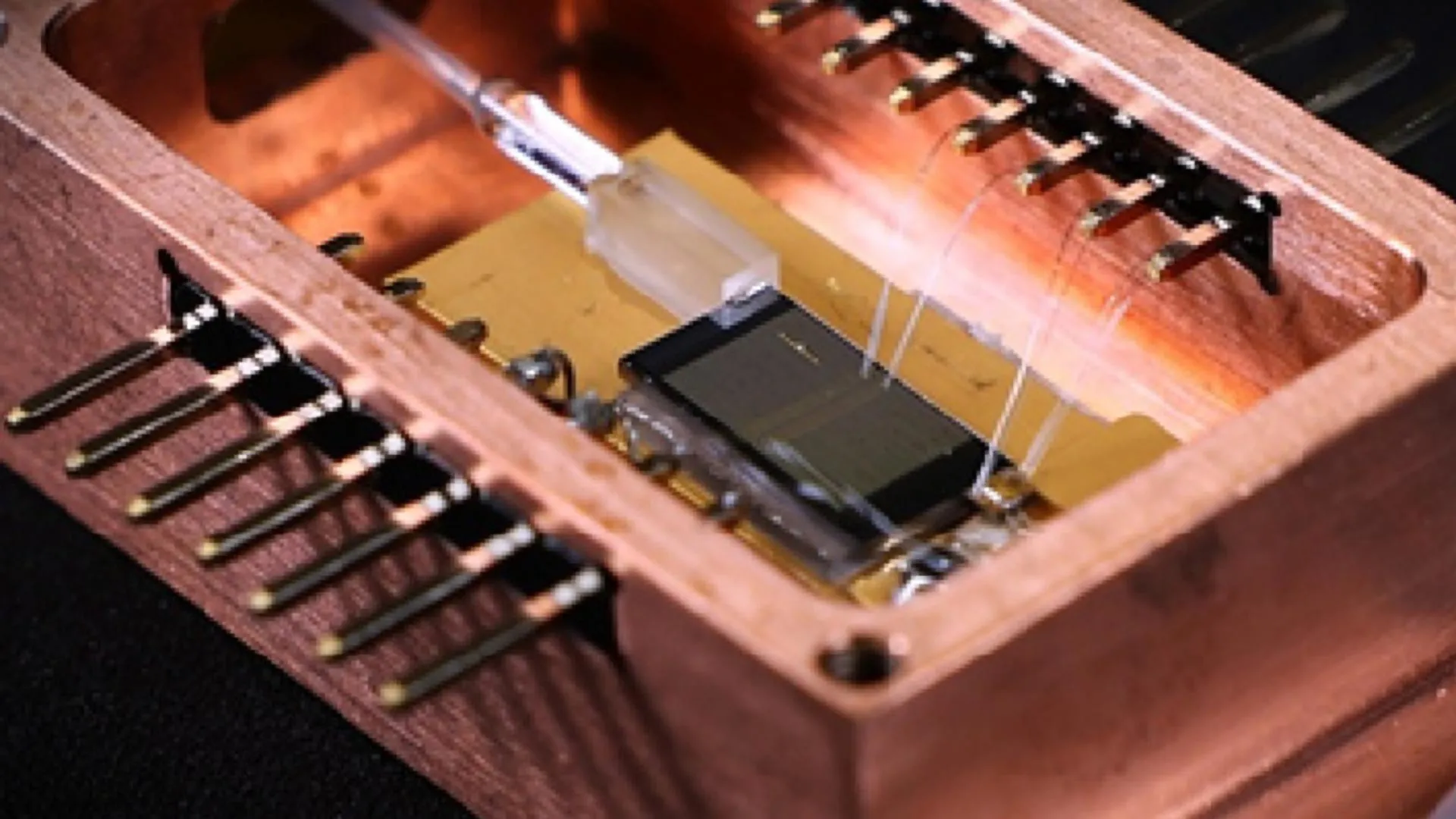
This tiny laser could transform how we see and sense the world
Laser technology plays a vital role in modern life, supporting everything from precise scientific measurements to advanced communication systems. It underpins technologies such as self-driving vehicles, high-speed fiber optic networks, and even…
Continue Reading
-
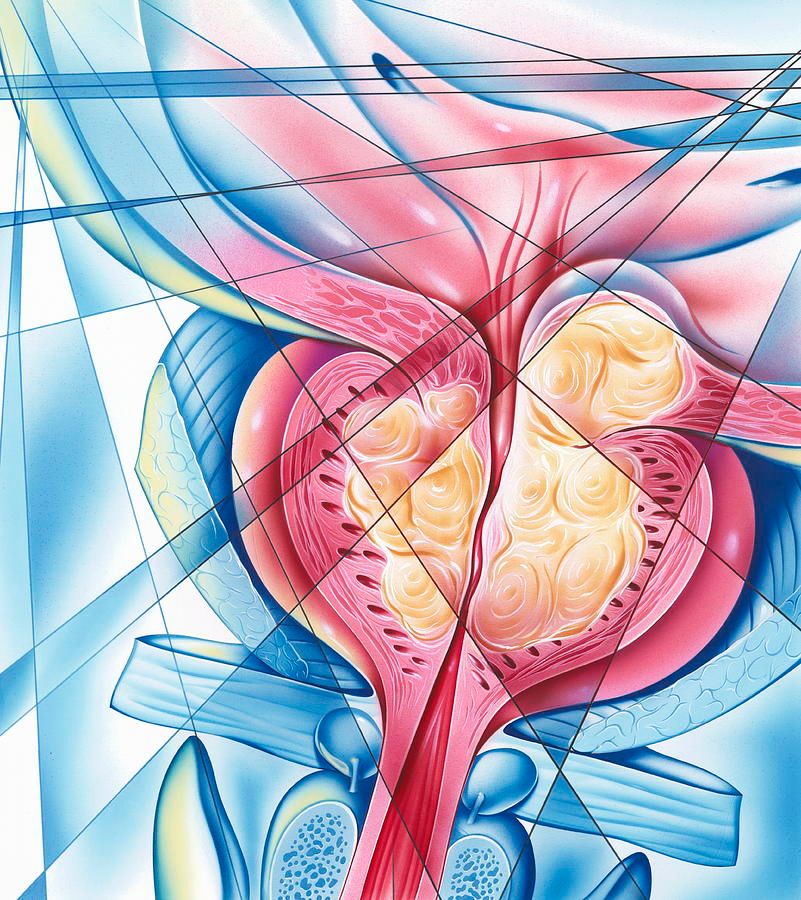
Capivasertib Combo Improves rPFS in PTEN-Deficient HSPC Population
Radiographic progression-free survival (rPFS) improved when adding capivasertib (Truqap) to abiraterone acetate, prednisone, and androgen deprivation therapy (ADT) among patients with PTEN-deficient de novo metastatic hormone-sensitive prostate cancer (HSPC), according to data from the phase 3 CAPItello-281 trial (NCT04493853) presented at the
European Society for Medical Oncology (ESMO) Congress 2025 .1After a median follow-up of 18.4 months, the median rPFS with the addition of capivasertib was 33.2 months (95% CI, 25.9-44.2) compared with 25.7 months (95% CI, 22.0-29.9) for placebo (HR, 0.81; 95% CI, 0.66-0.98; P = .034). Median overall survival (OS) was not yet reached at the time of the analysis for either arm. There had been 267 events at the time of the assessment, with 129 in the capivasertib arm and 138 in the placebo group (HR, 0.90; 95% CI, 0.71-1.15; P = .401).
“The primary end point was met, showing a statistically significant rPFS benefit with the combination of capivasertib and abiraterone, with consistent benefits observed across clinical end points,” lead investigator Karim Fizazi, MD, PhD, a medical oncologist at Institut Gustave Roussy and Centre Oscar Lambret, said during a presentation of the results. “The rPFS in the control arm was only 25.7 months, highlighting the poor prognosis of a PTEN population.”
In the CAPItello-281 trial, 1012 patients were treated with abiraterone acetate at 1000 mg with prednisone at 5 mg daily plus ADT. Patients were randomly selected to receive capivasertib at 400 mg twice daily for 4 days on and 3 days off (n = 507) or a matched placebo (n = 505). PTEN deficiency was defined as more than 90% of malignant cells with no specific cytoplasmic staining by IHC. Of those screened, approximately 25% matched these requirements.
The primary end point of the study was investigator-assessed rPFS. Secondary end points included overall survival (OS). The analysis presented at ESMO was the final analysis for rPFS. A final OS analysis is still planned.
The baseline characteristics were similar between groups. In the capivasertib and placebo arms, respectively, the median ages were 67 and 68 years. The total Gleason score was 8 or more for 78.5% and 79% of patients and the disease risk was high for 61.3% and 65.9% of those in the capivasertib and placebo groups, respectively. The primary location of disease metastases was the bone for 91.1% and 92.5% in the investigational and control arms, respectively. Nearly two-thirds of patients had an ECOG performance score of 0 with the remainder having a score of 1. Nearly three-fourths of patients had high-volume disease.
“Consistent with the poor prognosis of this PTEN-deficient prostate cancer population, most patients had high-risk, high-volume, high Gleason score disease,” said Fizazi.
In prespecified subgroup analyses, similar improvements in rPFS were seen with the addition of capivasertib, although none passed the bar for statistical significance. In those with high-volume disease with visceral metastases, the hazard ratio was 0.77 favoring the capivasertib arm (95% CI, 0.52-1.14) and in those with a Gleason score of 8 or more the hazard ratio was 0.82 (95% CI, 0.65-1.02). In those with a score lower than 8, the hazard ratio was 1.06 (95% CI, 0.66-1.69).
The time to next treatment was 37.0 months with capivasertib compared with 28.5 months with placebo (95% CI, 0.75-1.11). The median symptomatic skeletal event-free survival was 42.5 months with capivasertib compared with 37.3 months for placebo. Events for this end point included pathological fracture, spinal cord compression, use of radiation, surgical intervention, and death. There were 150 of these events in the capivasertib group and 176 in the placebo group (HR, 0.82; 95% CI, 0.66-1.02; P = .079).
“Roughly half of patients are still on drug and another analysis of overall survival is planned at another time cutoff,” Fizazi said. “There was a numerical improvement of 5.2 months prolongation in event-free survival in the capivasertib arm.”
The time to castration resistance was 29.5 months in the capivasertib group and 22.0 months for placebo (HR, 0.77; 95% CI, 0.63-0.94). For this study, Fizazi noted, castration resistance was defined as radiographic disease progression, PSA progression, and development of a skeletal event. The median time to PSA progression was not calculable at the time of the analysis, with 60 events recorded in the capivasertib arm compared with 82 in the placebo group (HR, 0.73; 95% CI, 0.52-1.01). Pain progression was still too early to measure, as too few events had occurred.
“Interestingly, the Kaplan-Meier curves for time to PSA progression defined by PCWG3 are much higher than those for time to castration resistance, indicating that many patients with PTEN loss tend to first experience a detrimental clinical event, such as an imaging-based progression or bone mobility, prior to a PSA rise of greater than 25%,” said Fizazi.
Additional analyses were completed looking at different PTEN expression levels, following the suggestion of an impact from other studies looking at AKT inhibitors. Of those with PTEN loss on 95% of cells or more, the median rPFS was 33.2 months with capivasertib and 22.7 months with placebo (HR, 0.75; 95% CI, 0.60-0.94). When PTEN loss was 99% or more, the median rPFS was 34.1 months with capivasertib vs 22.4 months for placebo (HR, 0.71; 95% CI, 0.52-0.97). When there was 100% PTEN loss, the median rPFS was 34.1 months compared with 22.1 months for capivasertib and placebo, respectively (HR, 0.68; 95% CI, 0.48-0.96).
“In the capivasertib arm, we see strongly consistent rPFS irrespective of the degree of PTEN deficiency; however, with increasing PTEN deficiency there is worsening prognosis in the control arm,” said Fizazi. “The same phenomenon of increasing treatment effect was also seen for overall survival.” For those with 100% PTEN loss, the early hazard ratio for OS was 0.77 (95% CI, 0.51-1.14).
The increase in treatment benefit was also seen across all secondary end points, when assessing higher levels of PTEN loss. At 100% PTEN loss, the hazard ratio for symptomatic skeletal event-free survival was 0.70 favoring capivasertib (95% CI, 0.48-1.01). For time to castration resistance the hazard ratio was 0.63 (95% CI, 0.45-0.89) and for time to PSA progression it was 0.55 (95% CI, 0.29-1.01). “It was exactly the same trend,” Fizazi said.
A grade 3 or higher adverse event (AE) was experienced by 67% of patients treated with capivasertib compared with for 40.4% of those in the placebo arm. A serious AE was experienced by 42.5% of those in the capivasertib group compared with for 26% of patients in the placebo arm. There was 36 AEs leading to death in the investigational arm compared with 26 in the placebo group.
Adverse events led to discontinuation of capivasertib or placebo for 18.3% and 4.8% of patients, respectively. For capivasertib and placebo, respectively, dose interruptions were required for 62.8% and 26.8% of patients and dose reductions were needed for 29% and 3.6% of patients. An AE led to discontinuation of abiraterone acetate for 9.5% and 5.4% of patients in the investigational and control arms, respectively.
The most common AEs in the capivasertib arm and placebo group, respectively, were diarrhea (51.9% vs 8.0%), hyperglycemia (38% vs 12.9%), rash (35.4% vs 7%), anemia (23.9% vs 12.7%), and hypokalemia (22.1% vs 12.7%). “Adverse events typical of abiraterone, such as hypertension, hypokalemia, and transaminase increase, were even between arms,” said Fizazi.
In early 2025,2 another study exploring capivasertib in prostate cancer was halted following an interim analysis that showed a potential lack of efficacy. This study, CAPItello-280 (NCT05348577), was exploring capivasertib in combination with docetaxel and ADT.
Outside of prostate cancer, capivasertib has gained FDA approval in combination with fulvestrant (Faslodex) for the treatment of patients with hormone receptor–positive, HER2-negative, locally advanced or metastatic breast cancer harboring 1 or more PIK3CA, AKT1, or PTEN alterations following progression on at least 1 endocrine-based regimen in the metastatic setting or recurrence on or within 12 months of completing adjuvant therapy.3
References
- Fizazi K, Clarke NW, De Santis M, et al. A phase III study of capivasertib (capi) + abiraterone (abi) vs placebo (pbo) + abi in patients (pts) with PTEN deficient de novo metastatic hormone-sensitive prostate cancer (mHSPC): CAPItello-281. Presented at: 2025 ESMO Congress; October 17-21, 2025; Berlin, Germany. Abstract 2383O.
- Update on CAPItello-280 phase III trial of Truqap in metastatic castration-resistant prostate cancer. News release. AstraZeneca. April 29, 2025. Accessed October 19, 2025. https://www.astrazeneca.com/media-centre/press-releases/2025/update-on-capitello-280-phase-iii-trial.html
- FDA approves capivasertib with fulvestrant for breast cancer. FDA. November 16, 2023. Accessed May 1, 2025. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-capivasertib-fulvestrant-breast-cancer
Continue Reading
-
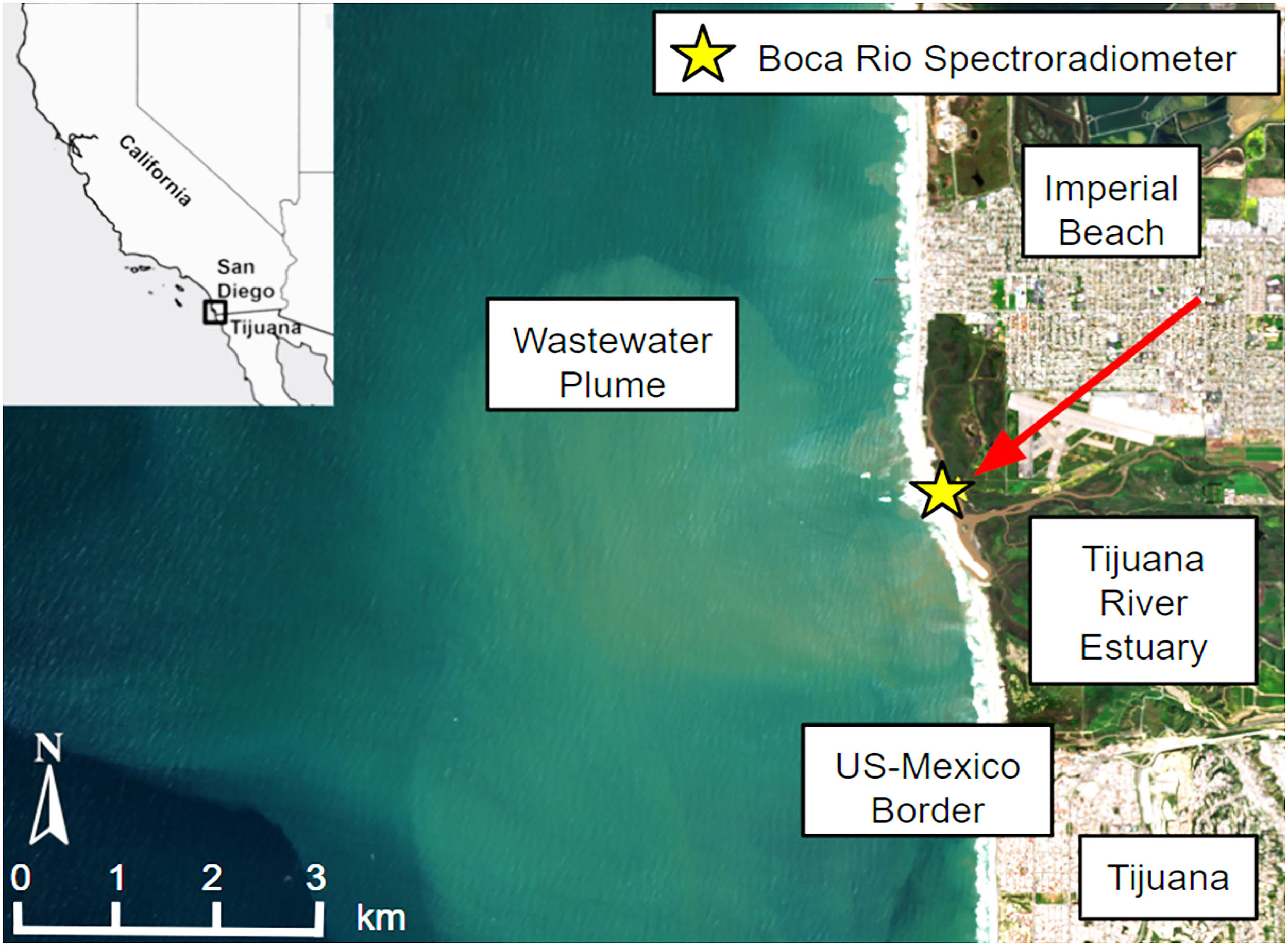
Satellite captures image of enormous sewage spill in coastal waters
NASA’s imaging spectrometer riding on the International Space Station has spotted a wastewater plume pouring from the Tijuana River into the Pacific. The signal is strong enough that scientists can track the pollution from orbit with a single…
Continue Reading
-
mPFS 11.14 Months (HR=0.6, P
- Ivonescimab plus chemotherapy demonstrated a median PFS of 11.14 months, PFS HR=0.60, P < 0.0001.
- The absolute difference in median PFS between the two groups was 4.24 months (ΔPFS = 4.24 months), indicating significantly prolonged progression-free survival with ivonescimab combination therapy.
- Significant benefits were consistently observed with ivonescimab plus chemotherapy versus tislelizumab combination irrespective of PD-L1 expression.
- Ivonescimab combination therapy showed significant benefit over tislelizumab regimen in patients with or without liver metastases and with or without brain metastases.
- In the HARMONi-6 study, 92.3% of enrolled patients had stage IV disease, and approximately 63% had central-type squamous cell carcinoma.
- Ivonescimab demonstrated a favorable overall safety profile with no new safety signals identified.
- Overall survival (OS) data were not yet mature at the time of this analysis.
HONG KONG, Oct. 19, 2025 /PRNewswire/ — Akeso (9926.HK) announced the groundbreaking results of the registrational Phase III clinical study (AK112-306/HARMONi-6) evaluating ivonescimab (PD-1/VEGF bispecific antibody), a globally first-in-class bispecific antibody developed by Akeso, in combination with chemotherapy versus tislelizumab combined with chemotherapy as first-line treatment for advanced squamous non-small cell lung cancer (sq-NSCLC). The study results were presented by Professor Lu Shun, Chief of the Shanghai Lung Cancer Center at Shanghai Chest Hospital and Professor of Medicine at Shanghai Jiaotong University, at the 2025 ESMO Presidential Symposium, and the results of the HARMONi-6 clinical trial were simultaneously published in The Lancet.
The HARMONi-6 study met its primary endpoint of progression-free survival (PFS), demonstrating a decisive and strong positive outcome with both statistically significant and clinically meaningful benefits. Ivonescimab plus chemotherapy substantially prolonged sq-NSCLC patient’s progression-free survival compared to tislelizumab plus chemotherapy:
- The progression-free survival (PFS) hazard ratio (HR) was 0.60 (p < 0.0001) for ivonescimab plus chemotherapy versus tislelizumab plus chemotherapy. The median PFS was 11.14 months in the ivonescimab combination group versus 6.90 months in the tislelizumab combination group, representing an absolute improvement of ΔPFS = 4.24 months.
- Consistent clinical benefits were observed across all PD-L1 expression subgroups. In the PD-L1 negative (TPS <1%) population, the PFS HR was 0.55 for ivonescimab plus chemotherapy versus tislelizumab plus chemotherapy. In the PD-L1 positive (TPS ≥1%) population, the PFS HR was 0.66 for ivonescimab plus chemotherapy versus tislelizumab plus chemotherapy.
- Regardless of liver or brain metastasis, ivonescimab combined with chemotherapy showed significant benefit over the tislelizumab-based regimen. Among patients with liver metastases, the PFS HR was 0.53; among those without liver metastases, the PFS HR was 0.64. For patients with ≥3 baseline metastatic sites, the PFS HR was 0.46, and for those with <3 baseline metastatic sites, the PFS HR was 0.64.
The ivonescimab group demonstrated a favorable overall safety profile with no new safety signals identified. The incidence of treatment-related serious adverse reactions and grade 3 or higher bleeding events was comparable to the tislelizumab regimen group. The most common adverse reactions were chemotherapy-associated myelosuppression.
The HARMONi-6 study enrolled 532 subjects, with balanced baseline characteristics between the two groups. 92.3% of subjects had clinical stage IV disease. The squamous carcinoma characteristics of enrolled patients reflected real-world clinical presentations, with central-type squamous carcinoma accounting for approximately 63% of the total patients (66.9% in the ivonescimab group vs. 59.4% in the tislelizumab group), consistent with real-world patient distribution. PD-L1 expression levels also reflected those seen in real-world clinical settings.
Approved in 2024, ivonescimab has demonstrated groundbreaking clinical value across dozens of clinical studies and real-world treatments involving over 40,000 patients. In the field of tumor immunotherapy, whether compared with PD-1 monotherapy or PD-1 combination chemotherapy (the current standard of care for many cancers), as well as in the field of anti-angiogenesis therapy compared to VEGF-targeted regimens, ivonescimab has demonstrated robust and superior clinical efficacy, highlighting its exceptional capacity to drive iterative advances in cancer treatment.
The encouraging results from the HARMONi-6 study have led to the review of a supplemental New Drug Application (sNDA) in China for ivonescimab in combination with chemotherapy as a first-line treatment for advanced squamous NSCLC. Meanwhile, the global enrollment for the international, multicenter Phase III HARMONi-3 trial, assessing ivonescimab as a first-line treatment for both squamous (sq-NSCLC) and non-squamous (nsq-NSCLC) non-small cell lung cancer is ongoing.
Forward-Looking Statement of Akeso, Inc.
This announcement by Akeso, Inc. (9926.HK, “Akeso”) contains “forward-looking statements”. These statements reflect the current beliefs and expectations of Akeso’s management and are subject to significant risks and uncertainties. These statements are not intended to form the basis of any investment decision or any decision to purchase securities of Akeso. There can be no assurance that the drug candidate(s) indicated in this announcement or Akeso’s other pipeline candidates will obtain the required regulatory approvals or achieve commercial success. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and health care legislation in P.R.China, the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; Akeso’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the Akeso’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
Akeso does not undertake any obligation to publicly revise these forward-looking statements to reflect events or circumstances after the date hereof, except as required by law.
About Akeso
Akeso (HKEX: 9926.HK) is a leading biopharmaceutical company committed to the research, development, manufacturing and commercialization of the world’s first or best-in-class innovative biological medicines. Founded in 2012, the company has created a unique integrated R&D innovation system with the comprehensive end-to-end drug development platform (ACE Platform) and bi-specific antibody drug development technology (Tetrabody) as the core, a GMP-compliant manufacturing system and a commercialization system with an advanced operation mode, and has gradually developed into a globally competitive biopharmaceutical company focused on innovative solutions. With fully integrated multi-functional platform, Akeso is internally working on a robust pipeline of over 50 innovative assets in the fields of cancer, autoimmune disease, inflammation, metabolic disease and other major diseases. Among them, 24 candidates have entered clinical trials (including 15 bispecific/multispecific antibodies and bispecific ADCs. Additionally, 7 new drugs are commercially available. Through efficient and breakthrough R&D innovation, Akeso always integrates superior global resources, develops the first-in-class and best-in-class new drugs, provides affordable therapeutic antibodies for patients worldwide, and continuously creates more commercial and social values to become a global leading biopharmaceutical enterprise.For more information, please visit https://www.akesobio.com/en/about-us/corporate-profile/ and follow us on Linkedin.
SOURCE Akeso, Inc.

Continue Reading
-

Former Big Brother contestant sues for disability after washing dishes on set
Former Big Brother contestant Shilo Shalom has filed a lawsuit against the National Insurance Institute, demanding recognition of what he claims is a lasting disability stemming from an injury he sustained while washing dishes during the 2024…
Continue Reading
-

‘Battlefield 6’ Season 1 release date, content, updates, and more
Battlefield 6 has had a stellar first week, becoming the most successful game in the franchise and EA has no plans to stop supporting the game as new content is planned before the end of the month.
- READ MORE: ‘Battlefield 6’ review: modern…
Continue Reading
-

Abadia Fall 2025 at Riyadh Fashion Week – WWD
- Abadia Fall 2025 at Riyadh Fashion Week WWD
- Vivienne Westwood Show Opens Riyadh Fashion Week, as Saudis Highlight Creative Side The New York Times
- Saudi labels ‘honor roots’ at Riyadh Fashion Week Arab News
- Mushroom skin bag and Riyadh…
Continue Reading