Ireland wing Tommy O’Brien said ahead of their Test in Chicago on Sunday (NZT) that while the All Blacks have their respect, they are no longer on a pedestal.
Since breaking their duck against the New Zealanders in their 2016…

Ireland wing Tommy O’Brien said ahead of their Test in Chicago on Sunday (NZT) that while the All Blacks have their respect, they are no longer on a pedestal.
Since breaking their duck against the New Zealanders in their 2016…
These differences don’t necessarily mean the AI-generated results are “worse,” of course. The researchers found that GPT-based searches were more likely to cite sources like corporate…
It sounds like the plot of a medieval historical drama: A once-powerful monarch, weakened by illness, is overthrown by her previously loyal subjects. But in honey bee colonies, such high-stakes coups aren’t just fantasy — they’re a…

Speakers from financial institutions, NGOs, government, and industry highlighted how biodiversity protection, community engagement, and energy development can be mutually reinforcing when built into project design and policy frameworks.
Examples ranged from solar projects in France that integrate wetland restoration, eco-grazing, and citizen investment, to marine wind farms in the North Sea linked to long-term marine conservation funding. In Uzbekistan, solar developers are protecting tortoise habitats and partnering with local herders to manage grazing. In Qinghai Province, China, large-scale PV parks are reversing desertification while supporting ecological animal husbandry.
Industry actors like TotalEnergies are scaling agro-photovoltaic models that combine renewable energy generation with sustainable farming. Policymakers and financial institutions, including the European Bank for Reconstruction and Development (EBRD) and the International Renewable Energy Agency (IRENA), underscored the role of strong policy frameworks, financing incentives, and capacity building to scale these approaches globally.
Ecowende is developing the Netherlands’ most ecological offshore wind farm to date—powering 3% of national demand while enhancing North Sea biodiversity—through an innovative, research-driven and collaborative approach supported by IUCN’s Biodiversity Advisory Team, which provides independent review and recommendations on biodiversity goals and targets.
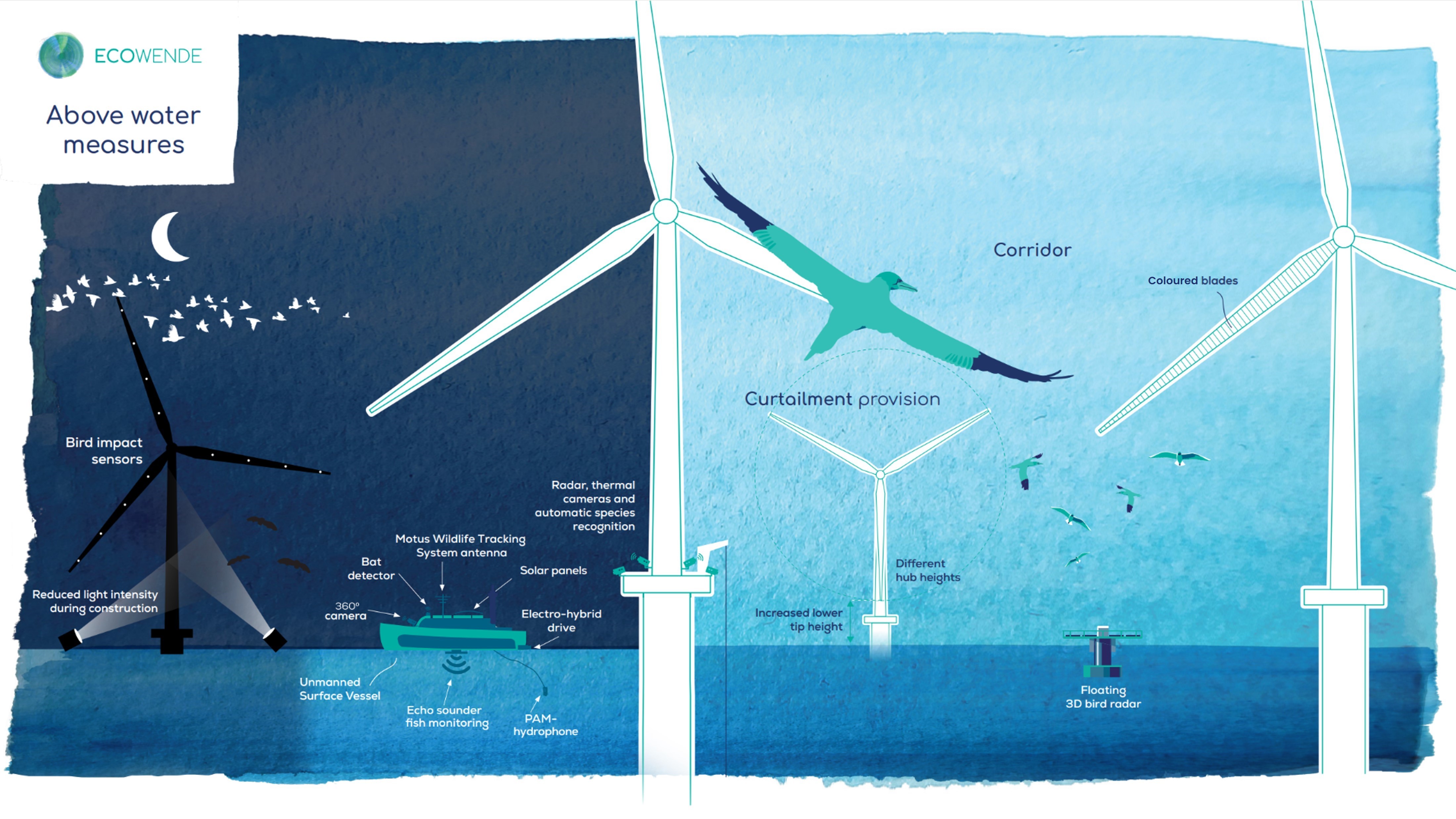
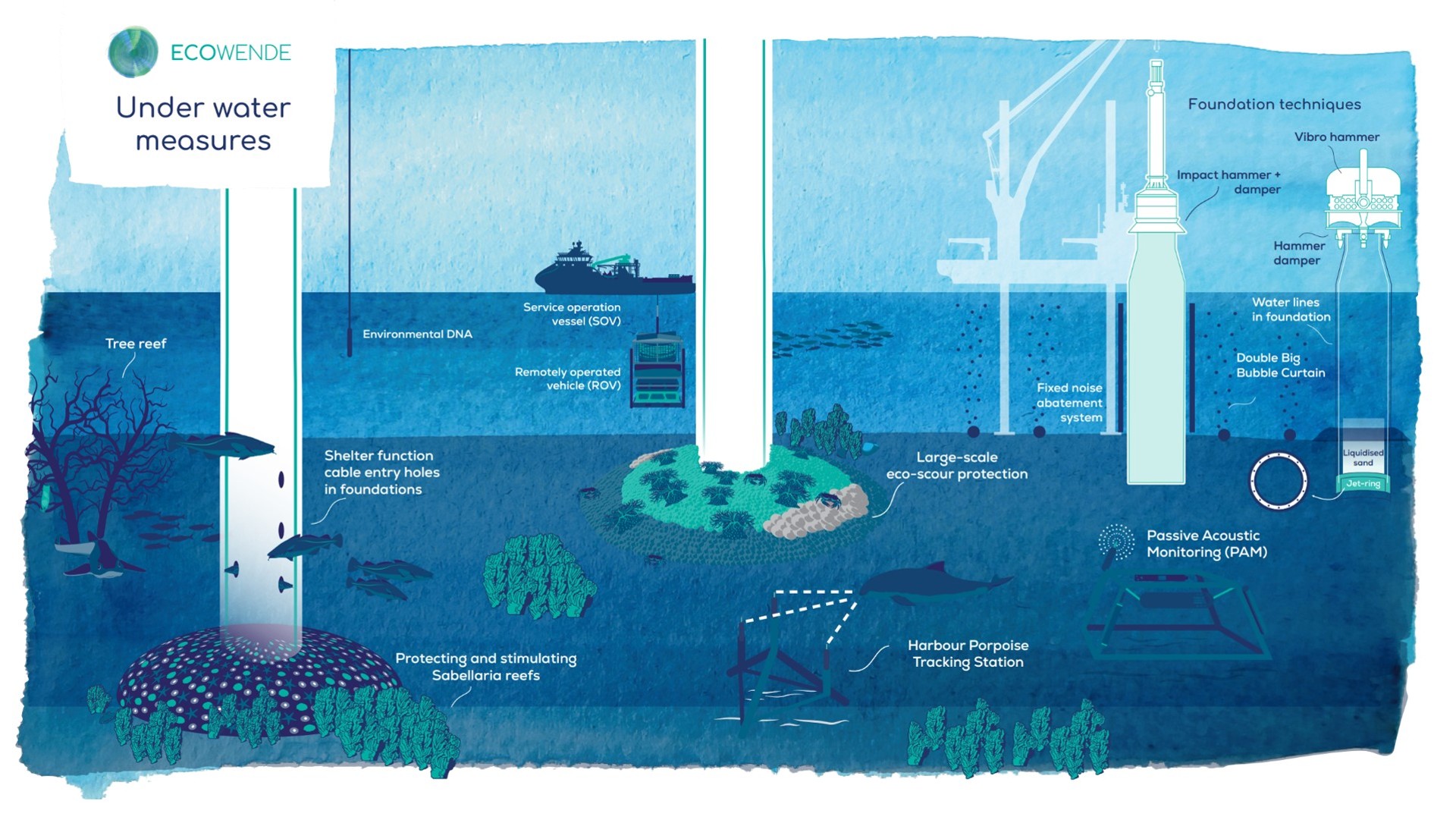
From regulatory tools to community partnerships, the session highlighted that the energy transition can—and must—deliver measurable gains for biodiversity and people.
The session was moderated by Qiulin Liu from IUCN and speakers included Adonai Herrera-Martínez from EBRD, Aonghais Cook from The Biodiversity Consultancy, Dr. Ma Hao from the Qinghai Provincial Development and Reform Commission, Jinlei Feng from IRENA, Karen Westley from Ipieca, Libby Sandbrook from Fauna & Flora, Sophie Depraz from Ipieca, Steven Dickinson from TotalEnergies, Yu Miao from SPIC Huanghe Hydropower Development Co., Ltd., and Zhang Jiali from the China Renewable Energy Engineering Institute (CREEI).

EXCLUSIVE: Josh Hartnett (Trap) will star in and produce the action-thriller All Day & All Night, written by Tommy Wirkola and John Niven and to be directed by Wirkola, the Norwegian filmmaker best known for the Violent Night and Dead Snow…
This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
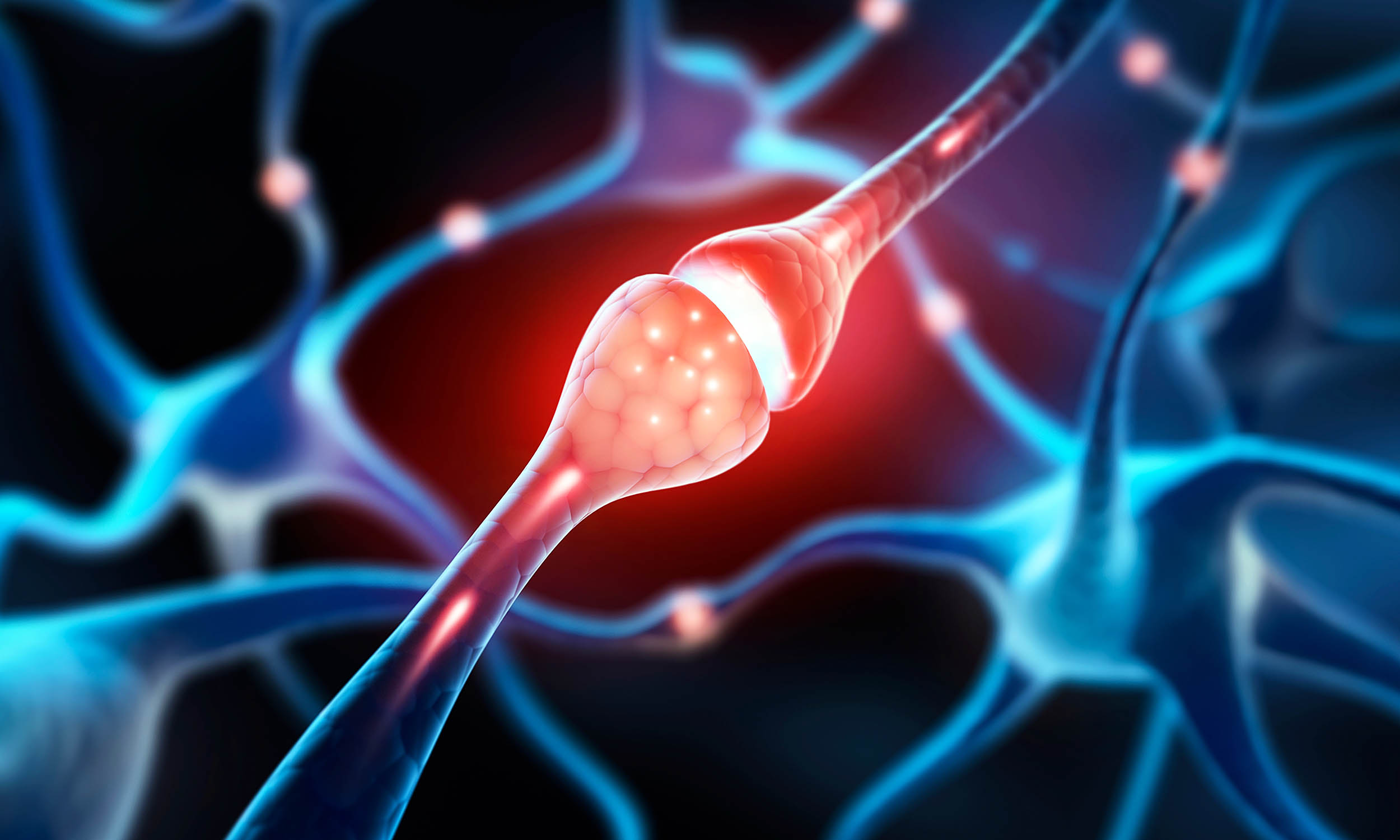
Brains run on timing. A fraction of a second can decide whether one message arrives before another and changes what a circuit does. Signals travel along axons – the thin extensions of brain cells (neurons) that act like wires.
Textbooks have…

Rihanna has long made her penchant for pink clear. Take one of her best maternity looks ever, a spring summer 2001 Issey Miyake dress made from semi-sheer pleats in a gradient of sorbet tones with a shoulder-engulfing neckline, and a breezy,…

Omvoh now offers patients a simplified maintenance experience with one monthly injection, replacing the previous two-injection regimen
Omvoh single-injection dosing will be available for U.S. patients in early 2026
This is the third FDA approval for Omvoh this year, following approvals for Crohn’s disease and a citrate-free formulation
INDIANAPOLIS, Oct. 27, 2025 /PRNewswire/ — Eli Lilly and Company (NYSE: LLY) announced today that the U.S. Food and Drug Administration (FDA) approved a single-injection, once-monthly maintenance regimen (200 mg/2 mL) of Omvoh (mirikizumab-mrkz) for subcutaneous use in adults with moderately to severely active ulcerative colitis (UC).
“In clinical practice, we see that simplifying maintenance treatment can make a difference in the overall patient experience,” said Miguel Regueiro, M.D., board-certified gastroenterologist specializing in inflammatory bowel disease. “A single monthly injection of Omvoh gives patients a regimen that’s easier to manage alongside the unpredictability of living with ulcerative colitis.”
The Omvoh single-injection, citrate-free maintenance dose will be available in the U.S. via prefilled pen or prefilled syringe in early 2026. The U.S. approval follows the recent European Union authorization of Omvoh for single-injection maintenance dosing for UC.
“People living with the constant discomfort and disruption caused by the symptoms of ulcerative colitis need treatments that offer the potential to achieve lasting remission and a convenient dosing option that fits easily into their lives,” said George Salem, M.D., director of Crohn’s and Colitis Center at OU HEALTH. “With this approval, patients who respond to induction therapy with Omvoh can continue maintenance therapy with the convenience of just one injection each month — delivering the same proven results with fewer injections.”
The single-injection approval is based on results from a Phase 1 study comparing one 200 mg/2 mL subcutaneous injection to two 100 mg/1 mL injections in participants. The study confirmed that Omvoh single-injection is bioequivalent to the previously approved two-injection regimen.1 Treatment with Omvoh for ulcerative colitis starts with 300-mg IV infusions every four weeks, for a total of three infusions, and at Week 12 transitions to subcutaneous self-injection every four weeks for maintenance treatment.
“At Lilly, we are committed to supporting people living with IBD by delivering meaningful clinical outcomes and continuing to improve their treatment experience,” said Ashley Diaz-Granados, senior vice president of U.S. Immunology at Lilly. “Building on the introduction of a citrate-free formulation of Omvoh earlier this year, this approval further delivers on our commitment by providing patients the same outcomes in a single-injection maintenance regimen that fits more seamlessly into their lives.”
Omvoh is approved in the U.S. for the treatment of moderately to severely active UC and moderately to severely active Crohn’s disease in adults and has been approved in 45 countries around the world. Through Lilly Support Services™, Lilly offers a patient support program including co-pay assistance for eligible, commercially insured patients.
INDICATIONS AND SAFETY SUMMARY
Omvoh® (ahm-VOH) is a medicine used to treat
It is not known if Omvoh is safe and effective in children under 18 years of age.
Warnings – Omvoh can cause serious side effects including:
Serious allergic reactions: Omvoh may cause serious allergic reactions that may need to be treated in a hospital and may be life-threatening. Do not use Omvoh if you have had a serious allergic reaction to mirikizumab-mrkz or any of the ingredients in Omvoh. See the Medication Guide that comes with Omvoh for a list of ingredients. Stop using Omvoh and get emergency medical help right away if you develop any of the following symptoms of a serious allergic reaction:
Infections: Omvoh may lower the ability of your immune system to fight infections and may increase your risk of infections. If you have an infection, your healthcare provider should not start treatment with Omvoh until your infection is gone. Before starting treatment with Omvoh, your healthcare provider should assess you for tuberculosis (TB). If you are at risk for TB, you may be treated with medicine for TB before you begin treatment with Omvoh. Your healthcare provider should watch you closely for signs and symptoms of TB while you are being treated with Omvoh and after treatment.
Before starting Omvoh, tell your healthcare provider if you think you have an infection or have symptoms of an infection, such as:
After starting Omvoh, tell your healthcare provider right away if you have any symptoms of an infection.
Liver Problems: Omvoh may cause liver problems. Your healthcare provider should do blood tests to check your liver enzyme and bilirubin levels before treatment, during, and after treatment with Omvoh. Your healthcare provider may hold or stop treatment if needed. Tell your healthcare provider right away if you develop any signs and symptoms of liver problems, including:
Common side effects
The most common side effects of Omvoh in people treated for ulcerative colitis include:
The most common side effects of Omvoh in people treated for Crohn’s disease include:
These are not all the possible side effects of Omvoh.
Tell your doctor if you have any side effects. You can report side effects at 1-800-FDA-1088 or www.fda.gov/medwatch.
Before you use Omvoh, review these questions with your doctor:
Tell your doctor about all your medical conditions, including if:
How to take
Follow your healthcare provider’s instructions for using Omvoh. You will receive your first 3 doses of Omvoh through a vein in your arm (intravenous infusion) in a healthcare facility by a healthcare provider every 4 weeks. Each infusion will last about 30 minutes (for ulcerative colitis) or about 90 minutes (for Crohn’s disease). After induction, you will continue to receive Omvoh maintenance doses as self-injections under the skin (subcutaneous injection) every 4 weeks. For these injections, Omvoh is available as prefilled pens or prefilled syringes. (If taking Omvoh for Crohn’s disease, you will need two injections to complete your dose, using either two prefilled pens or two prefilled syringes.) If you give injections at home, you should be trained on the correct way to prepare and inject Omvoh. Do not try to inject Omvoh yourself until you or your caregiver have been shown how to inject. Read the detailed Instructions for Use about how to use and dispose of Omvoh the correct way.
Learn more
Omvoh is a prescription medicine. During induction, Omvoh is available as a single-dose vial for intravenous infusion containing 300 mg/15 mL that is administered in a healthcare facility.
During maintenance, Omvoh is available as:
For more information, call 1-800-545-5979 or go to omvoh.lilly.com.
This summary provides basic information about Omvoh but does not include all information known about this medicine. Read the information that comes with your prescription each time your prescription is filled. This information does not take the place of talking with your doctor. Be sure to talk to your doctor or other healthcare provider about Omvoh and how to take it. Your doctor is the best person to help you decide if Omvoh is right for you.
MR CON BS 24OCT2025
Omvoh® and its delivery device base are trademarks owned or licensed by Eli Lilly and Company, its subsidiaries, or affiliates.
About Omvoh
Omvoh (mirikizumab-mrkz) is an interleukin-23p19 (IL-23p19) antagonist indicated for the treatment of moderately to severely active ulcerative colitis and Crohn’s disease in adults. Omvoh selectively targets the p19 subunit of IL-23 and inhibits the IL-23 pathway. Inflammation due to over-activation of the IL-23 pathway plays a critical role in the pathogenesis of inflammatory bowel disease.2
Omvoh and its delivery device base are trademarks owned by Eli Lilly and Company.
About Lilly
Lilly is a medicine company turning science into healing to make life better for people around the world. We’ve been pioneering life-changing discoveries for nearly 150 years, and today our medicines help tens of millions of people across the globe. Harnessing the power of biotechnology, chemistry and genetic medicine, our scientists are urgently advancing new discoveries to solve some of the world’s most significant health challenges: redefining diabetes care; treating obesity and curtailing its most devastating long-term effects; advancing the fight against Alzheimer’s disease; providing solutions to some of the most debilitating immune system disorders; and transforming the most difficult-to-treat cancers into manageable diseases. With each step toward a healthier world, we’re motivated by one thing: making life better for millions more people. That includes delivering innovative clinical trials that reflect the diversity of our world and working to ensure our medicines are accessible and affordable. To learn more, visit Lilly.com and Lilly.com/news, or follow us on Facebook, Instagram, and LinkedIn. P-LLY
CMAT-02499 10/2025 © Lilly USA, LLC 2025. ALL RIGHTS RESERVED.
Trademarks and Trade Names
All trademarks or trade names referred to in this press release are the property of the company, or, to the extent trademarks or trade names belonging to other companies are references in this press release, the property of their respective owners. Solely for convenience, the trademarks and trade names in this press release are referred to without the ® and ™ symbols, but such references should not be construed as any indicator that the company or, to the extent applicable, their respective owners will not assert, to the fullest extent under applicable law, the company’s or their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements (as that term is defined in the Private Securities Litigation Reform Act of 1995) about Omvoh (mirikizumab-mrkz) as a treatment for people with moderate to severe ulcerative colitis and moderate to severe Crohn’s disease and reflects Lilly’s current beliefs and expectations. However, as with any pharmaceutical product, there are substantial risks and uncertainties in the process of drug research, development, and commercialization. Among other things, there is no guarantee that planned or ongoing studies will be completed as planned, that future study results will be consistent with study results to date, that Omvoh will receive additional regulatory approvals, or that Omvoh will be commercially successful. For further discussion of these and other risks and uncertainties that could cause actual results to differ from Lilly’s expectations, see Lilly’s Form 10-K and Form 10-Q filings with the United States Securities and Exchange Commission. Except as required by law, Lilly undertakes no duty to update forward-looking statements to reflect events after the date of this release.
References
1 Otani Y, et al. One subcutaneous injection of mirikizumab is bioequivalent to two subcutaneous injections: results from a pharmacokinetic comparability study in healthy participants. 2025 United European Gastroenterology Week. October 4-7, 2025.
2 Omvoh. Prescribing Information. Lilly USA, LLC.
Refer to: Kelly Hoffman; [email protected]; 765-736-2555 (Lilly media)
Michael Czapar; [email protected]; 317-617-0983 (Investors)
SOURCE Eli Lilly and Company