Microtubular transport of different organelles within the lysosomal system is essential for their proper function. Among the small GTPases that regulate late endosome/lysosome positioning, Rab7 (Fujiwara et al., 2016; Jordens et al., 2001; Ma et al., 2018; van der Kant et al., 2013) and Arl8 (Bagshaw et al., 2006; Boda et al., 2019; Hofmann and Munro, 2006; Marwaha et al., 2017; Rosa-Ferreira and Munro, 2011; Rosa-Ferreira et al., 2018) are well-studied. However, the positioning of autophagosomes is less understood. Autophagosome transport has been primarily studied in highly polarized neurons under basal conditions. In neuronal cells, dynein-mediated transport, regulated by several suggested adaptor molecules, has been described. However, most reporters and reagents used for studying autophagosome transport cannot differentiate between pre-fusion and post-fusion autophagic vesicles. Therefore, the regulation of non-fused autophagosome motility remains unclear. We propose that an autophagosome should not be considered as such once its lumen has started to acidify or undergo fusion, transitioning to a maturing autolysosome.
To address this issue, we established a genetic system generating mosaic cells where Vps16A, a central HOPS subunit, is silenced. Without Vps16A, cells are unable to fuse autophagosomes with lysosomes or late endosomes (Takáts et al., 2014), thus preventing the formation of autolysosomes. This system allowed us to study autophagosome positioning without misidentifying autolysosomes as autophagosomes. Given that fat cells have perinuclear non-centrosomal MTOCs, we hypothesized that non-fused autophagosomes move in a minus-end direction, likely driven by dyneins. This hypothesis was supported by our observation that relocating the ncMTOC using shot RNAi also relocated autophagosomes to this region.
Loss of dyneins and dynactins not only blocked perinuclear autophagosome positioning but also redistributed them to the cell periphery, suggesting that kinesin-regulated motility becomes available for starvation-induced autophagosomes in these conditions. Importantly, we showed that not all dyneins or dynactins are required for this transport. A dedicated cytosolic dynein complex, composed of Dhc64 (a heavy chain subunit), sw (an intermediate chain subunit), Dlic (a light intermediate chain subunit), and robl (a light chain subunit), activated by a dynactin complex, is required for autophagosome transport. This is consistent with observations that autophagosomes can move bidirectionally in neurons and that purified autophagosome fractions contain both dyneins and kinesins (Maday et al., 2012). Our results also reinforce findings that microtubule inhibitors block centrosome-directed autophagosome transport in mammalian cells (Fass et al., 2006).
Taking these observations into consideration, we investigated the relationship between dyneins and kinesins in autophagosome transport. Overexpression of kinesin motors blocked minus-end transport, while autophagosomes in kinesin and dynactin double knockdown cells appeared immobile. Moreover, expressing a recombinant minus-end kinesin (Clark et al., 1997) partially rescued the peripheral relocation of autophagosomes upon dynactin silencing, indicating a competitive relationship between minus- and plus-end motors in autophagosome positioning. The possibility of plus-end transport as a secondary mechanism raises questions about its physiological role. Besides enabling bidirectional movement (Jahreiss et al., 2008; Maday et al., 2012), it is possible that autophagosomes transport kinesins to autolysosomes, similar to endosomes, which are suggested to transport dyneins to autophagic vacuoles (Cheng et al., 2015). Therefore, if kinesins are present but are downregulated on autophagosomes, the absence of dyneins could potentially release them from inhibition. Autophagic vesicles are suggested to move towards the plus end (Mauvezin et al., 2016; Pankiv et al., 2010), and loss of various kinesins leads to autophagosome accumulation in the cell center in mammalian cells (Cardoso et al., 2009; Korolchuk et al., 2011). The plus-end transport of autophagic vesicles by the Klp98A kinesin in Drosophila promotes autophagosome-lysosome fusion and degradation (Mauvezin et al., 2016). However, our tests indicated that Rab14 and its interactor Klp98A likely transport autolysosomes, not autophagosomes, as evidenced by the non-autophagosomal localization of YFP-Rab14 and that their RNAi-s had no effect on autophagosome positioning in starved, HOPS-depleted cells. Since neither kinesin RNAi altered the perinuclear accumulation of autophagosomes in Vps16A-depleted cells, we propose that the default direction is towards the MTOC at the minus-ends of microtubules.
Among Rab small GTPases, we identified Rab7 and Rab39 as regulators of autophagosome positioning. Rab7, crucial for autophagosome-lysosome fusion, appears on autophagosomes (Hegedűs et al., 2016). Its knockdown resulted in autophagosomes remaining randomly positioned in the cytosol, highlighting its importance in bidirectional motility. Consistent with our findings, Rab7 regulates both minus- and plus-end directed motility of lysosomes or endosomes, involving several adaptors such as Plekhm1 and FYCO1 (Fujiwara et al., 2016; Jordens et al., 2001; Ma et al., 2018; Pankiv et al., 2010; Tabata et al., 2010; van der Kant et al., 2013). However, only one Rab7 interactor, the autophagy adaptor Epg5 (Gillingham et al., 2014; Wang et al., 2016), produced a similar phenotype to Rab7 in our tests. Additionally, we demonstrated that Epg5 coprecipitates with Dhc64C, and localizes to Rab7 and Atg8a-positive vesicles in cultured fruit fly cells. As Epg5 loss did not significantly impact the endolysosomal system, it appears to function in the autophagic pathway. Epg5 interacts with Rab7 and LC3 to mediate autophagosome-lysosome fusion in fly, worm, and mammalian cells (Hori et al., 2017; Wang et al., 2016), and its mutations are linked to Vici syndrome, a severe neurodegenerative disorder in humans (Balasubramaniam et al., 2018; Byrne et al., 2016; Meneghetti et al., 2019). We thus identify a potential new role for Epg5 in autophagosome positioning.
Silencing Rab39 and its interactor ema (Gillingham et al., 2014) produced a phenotype similar to Rab7 or Epg5 loss. Rab39 regulates lysosomal function and interacts with HOPS in mammalian cells (Lakatos et al., 2021; Zhang et al., 2023). Ema promotes autophagosome biogenesis (Kim et al., 2012) and endosomal maturation through HOPS interaction (Kim et al., 2010). Our results suggest that Rab7-Epg5 and Rab39-ema interactions are both necessary for bidirectional autophagosome motility. Further studies are required to clarify their exact roles and interrelations. YFP-tagged Rab7 and Rab39 both colocalized with autophagosomes, supporting their role in motility. Rab2, Rab7, and Arl8 are known lysosomal fusion factors (Boda et al., 2019; Hegedűs et al., 2016; Lőrincz et al., 2017b), but unlike Rab7, neither Rab2 nor Arl8 knockdown affected autophagosome positioning. Since YFP-Rab2 was also found to colocalize with autophagosomes, our results suggest that its sole role on autophagosomes is to regulate maturation. Although our RNAi screen identified a molecular machinery potentially sufficient for autophagosome transport, other small GTPases, adaptors, or motor subunits may also influence this process. Because validating the proper loss-of-function effect for every RNAi line used in the screen was impractical, we cannot exclude the possibility that some negative results were due to inefficient silencing. It is also important to note that Drosophila cells exhibit a wide variety of microtubule-organizing centers (MTOCs) during development, including both centrosomal and non-centrosomal types (Tillery et al., 2018). A key future direction will be to examine autophagosome motility in additional cell types to determine whether the same positioning machinery operates universally, or whether cell-type–specific mechanisms exist.
Given the small Arl8-positive lysosomes in the perinuclear region upon Vps16A silencing, we hypothesized that pre-fusion lysosomes might exhibit similar motility to autophagosomes. This was largely confirmed, as minus-end transport of immature lysosomes depended on dyneins, Rab7, Rab39, and ema. However, Epg5 did not influence lysosome positioning, indicating that its function is likely exerted at the surface of autophagosomes. Conversely, Rab2 was identified as a regulator of minus-end-directed lysosome transport. These findings suggest similar but distinct regulatory mechanisms for pre-fusion organelle transport, possibly to enhance fusion efficiency by converging them towards the cell center. Rab2’s interaction with motor adaptors such as Bicaudal D (Gillingham et al., 2014) likely regulates lysosome motility.
Moreover, mature autolysosomes, but not pre-fusion ones, redistributed to the cell periphery upon Rab39 silencing, similar to dynein-depleted cells, suggesting that Rab39 exclusively regulates minus-end movement at the post-fusion level. Considering that plus-end directed lysosome motility is regulated by Arl8 (Bagshaw et al., 2006; Boda et al., 2019; Hofmann and Munro, 2006; Marwaha et al., 2017; Rosa-Ferreira and Munro, 2011; Rosa-Ferreira et al., 2018), which is dispensable for autophagosome motility, it is plausible that Rab39 promotes bidirectional transport of pre-fusion organelles, while its role post-fusion is restricted to minus-end directed motility.
An important question is why pre-fusion autophagosomes and lysosomes travel to the same destination. It is feasible to think this is because they need to fuse with each other. We found that loss of minus-end directed motility reduced autolysosome maturation; in dynein- or dynactin-depleted cells, only smaller autolysosomes were produced, and autophagosome-lysosome fusion decreased. This aligns with previous observations that dynein-regulated autophagosomal motility is indispensable for efficient lysosomal fusion (Jahreiss et al., 2008; Kimura et al., 2008). Conversely, inhibiting kinesins did not impede autophagosome-lysosome fusion, and concentrating autophagosomes and lysosomes to a smaller ectopic ncMTOC via shot RNAi resulted in enlarged lysosomes, allowing autophagosome-lysosome fusion to proceed.
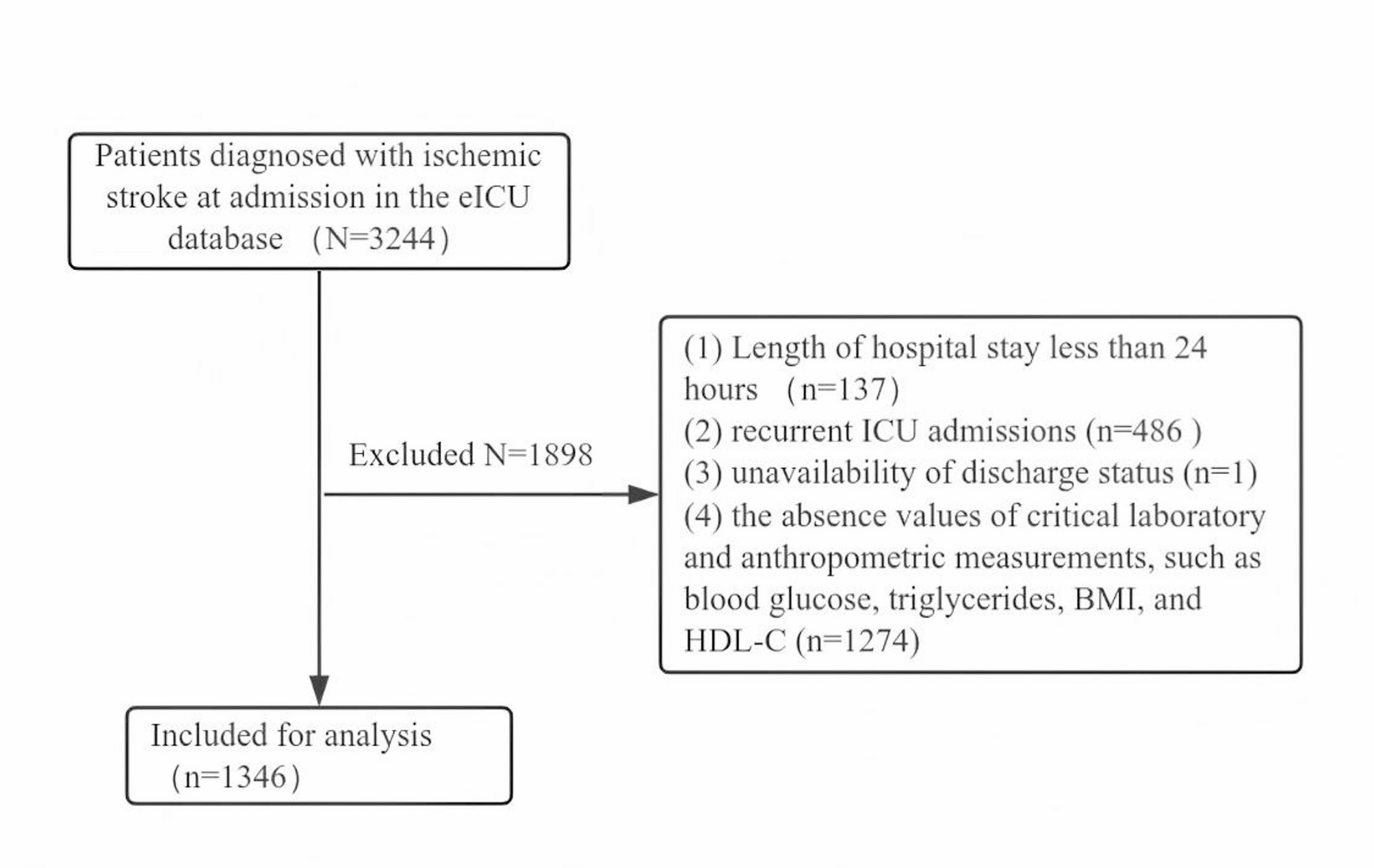
Therefore, we propose a model in which pre-fusion organelles travel towards the MTOC in a cytosolic dynein-dependent manner regulated by small GTPases and their adaptors (Rab7-Epg5 and Rab39-ema on autophagosomes; Rab7, Rab39-ema, and Rab2 on lysosomes) to enhance fusion probability. After fusion, autolysosomes can move to the periphery in an Arl8-dependent manner and back, regulated by Rab39 (Figure 11).
Model of the transport of autophagosomes and lysosomes in starved fat cells.
Before fusion, autophagosomes and lysosomes are transported towards the perinuclear non-centrosomal microtubule organizing center (ncMTOC) by a cytosolic dynein complex in starved fat cells to ensure proper fusion and effective degradation. This process requires Rab7, Rab39, and their interactors Epg5 and ema on autophagosomes, and Rab2, Rab7, Rab39, and the Rab39 interactor ema, but not Epg5, on lysosomes. After fusion, Arl8 mediates the plus-end transport of autolysosomes, while Rab39 promotes dynein-regulated minus-end directed transport. Thus, the motility of pre-fusion and post-fusion organelles is differently regulated: pre-fusion organelles generally move towards the MTOC, while post-fusion organelles exhibit bidirectional motility. This spatial regulation ensures proper fusion rates and degradation efficiency.