Model approach
The base structure of this model was adapted from the PROMETHEUS study conducted in the United States based [15]. Our multi-year stochastic microsimulation model projects outcomes over 10-years by starting with a base population of Italian COPD patients and incorporating new entrants (incident cases) annually. Literature-based assumptions were generated to create a population that represents the current Italian COPD population by assigning characteristics such as age, gender, COPD incidence rates, yearly changes in airflow restriction, baseline treatments as well as treatment modifications, mortality rates, and exacerbation rates (for both moderate and severe exacerbations). We describe the literature sources used for each characteristic in the supplemental materials. If an Italian source was available, it was used for inclusion with preference given to sources that were more recent in order to best represent the current Italian COPD population. In cases where there was no Italian data source, we utilized other available data as outlined. The percent of predicted forced exhalation volume in 1 s (FEV1) was then utilized to assign patients to a GOLD stage, 1–4. We then applied annual declines in FEV1 to model the progressive nature of the disease. Additional penalties were also applied if a patient was deemed to be a smoker.
A baseline (or status quo) model was created by integrating population growth in accordance with the projected increase of the Italian population over the 10-year forecast period.
Simulation overview, model populations, and simulation scenarios
After defining the baseline “Status Quo” model, we modeled 1,000 simulations over 10 years from 2025 to 2034. Each year, we applied probabilistic changes to the COPD patient characteristics. Changes included FEV1 decline, as mentioned above, smoking cessation rates, changes in COPD treatments, all-cause mortality, and occurrence of moderate and severe exacerbations. Any patient that did not quit smoking or those that experienced exacerbations was given a higher probability of COPD progression/FEV1 decline, which is in line with disease pathophysiology. We also linked medication therapy utilization to Exacerbations, based on available data on varying Exacerbation rates among patients on different therapies. Lastly, in addition to annually adding newly diagnosed incident COPD patients, patients were removed from the model either by dying or by reaching 100 years of age.
We modeled two scenarios – “Status Quo” and “Increased SITT”.
-
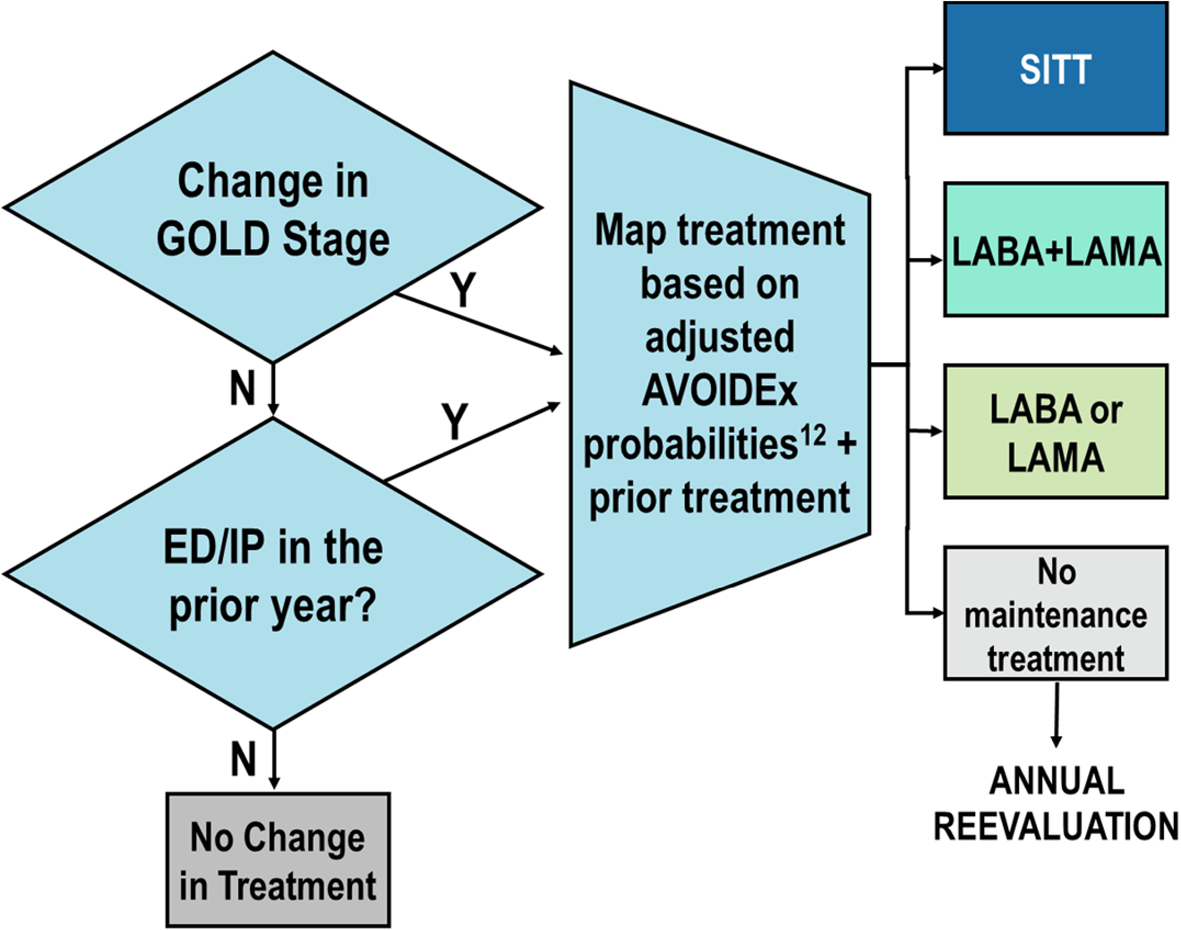
“Status Quo” (Baseline): this model was a simulation of the current Italian COPD population without applying any changes in current SITT prescribing patterns (Fig. 1).
-
“Increased SITT”: this model assumed a higher SITT prescribing practice occurring over the modeled 10-years based on GOLD prescribing guidelines (Fig. 2). The key difference between the “Increased SITT” and the “Status Quo” modeled populations was that the “Increased SITT” model modified the annual medication algorithm, resulting in a higher transition of patients to SITT.
We utilized data from the AVOIDEx study to map the probability of treatment changes in our status quo population [16]. Our treatment algorithm is based on both GOLD guidelines as well as key inclusion criteria from the ETHOS study. Our results are reported for two different populations within each scenario: the Total and Flagged populations. The flagged population represents the subset of the total population that met GOLD recommendation criteria for SITT prescribing, independent of the actual modeled medication use for those patients. These patients represent the more severe subset within the total population. Both the total and flagged populations were sampled and simulated separately from each other and therefore there may be variation in the patient counts and outcomes for the non-flagged population. The exacerbation rates were an important assumption in our modeling. To determine the baseline rate, we utilized the Gulp study and then utilized additional literature to determine the difference in rates [12, 17]. This approach was used to avoid over emphasizing the benefits of SITT compared to MITT in a real-world population on the exacerbation rate.
“Status Quo” medication transition
If a patient started on SITT, no further transitions were made, ED emergency department, IP inpatient admission, SITT single inhaler triple therapy, LABA long-acting beta 2- agonists, LAMA long-acting anticholinergics
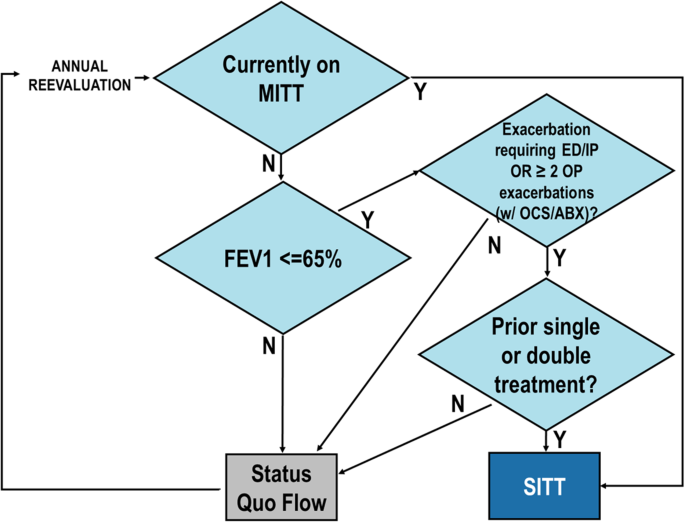
“Increased SITT” medication transition based on GOLD 2023 guidelines and key inclusion criteria for ETHOS study
If a patient started on SITT, no further transitions were made MITT multiple inhaler triple therapy, FEV1 forced expiratory volume in 1 s, ED emergency department, IP inpatient admission, OP outpatient visit, OCS oral corticosteroids, ABX antibiotics, SITT single inhaler triple therapy
We estimated the number of life-years extended for both the total and flagged populations under higher SITT adoption scenarios compared to status quo. Initially, we calculated the expected years of life by using standard mortality tables and considering the age and sex distribution. Subsequently, we adjusted the standard mortality rates to reflect the increased mortality associated with COPD and estimated the expected future years of life for each population.
Outcomes and statistical analyses
Over our 10-year model, we determined the simulated severe and moderate exacerbation rates and counts as well as the all-cause mortality rate and death counts for both the total population as well as the flagged population under both the “Status Quo” and the “Increased SITT” models. We also calculate the change in mortality, the life years extended and the difference in the exacerbation rates and counts between both models (for both the total and flagged populations, separately). Of note, the flagged population drives much of the numerical difference in mortality and morbidity observed in the total population. Lastly, we calculated the number needed to treat (NNT) to increase life expectancy by one year for the “Increased SITT” model. We also calculated the number of remaining life years in the flagged population under both the “Increased SITT” and “Status Quo” models over a 10 year-period. This was done to quantify the impact of increased SITT on the life expectancy of each COPD patient.
We utilized Python PySpark and the following statistical analyses were included: (1) use of a stochastic microsimulation model, (2) descriptive statistics including means for continuous variables, and (3) frequency percentages for categorical variables were calculated.
Sensitivity analyses
To assess the impact of variations in our baseline assumptions on modeled outcomes, we performed sensitivity testing on four variables. We varied: (1) the baseline distribution of GOLD airflow limitation stages, (2) the exacerbation rates, (3) the COPD population growth, and (4) the FEV1% of predicted cutoff required to consider patients for SITT therapy under the “Increased SITT” model and observed the impact on mortality, exacerbation counts and costs.
For the Baseline GOLD distribution, we either assumed that the Italian COPD population was 10% more or 10% less severe than the current assumption by shifting more/less patients to a higher or lower GOLD stage, respectively. In the scenario where we shifted severity up by 10%, we had a higher distribution of patients in higher GOLD stages than in the base model, and vice-versa for the 10% less severe scenario. Regarding Exacerbation rates, we assumed that the Exacerbation rate was either 10% higher or lower than the Baseline assumption. For the COPD incidence, we assumed that the rate was either 1% higher or 1% lower than the base case assumption used. For the FEV1% of predicted cutoff, we modified the 65% cutoff to 80% in the “Increased SITT” model. This latter assumption is more aligned to the label of triple inhaled therapies and GOLD guidelines in 2024.