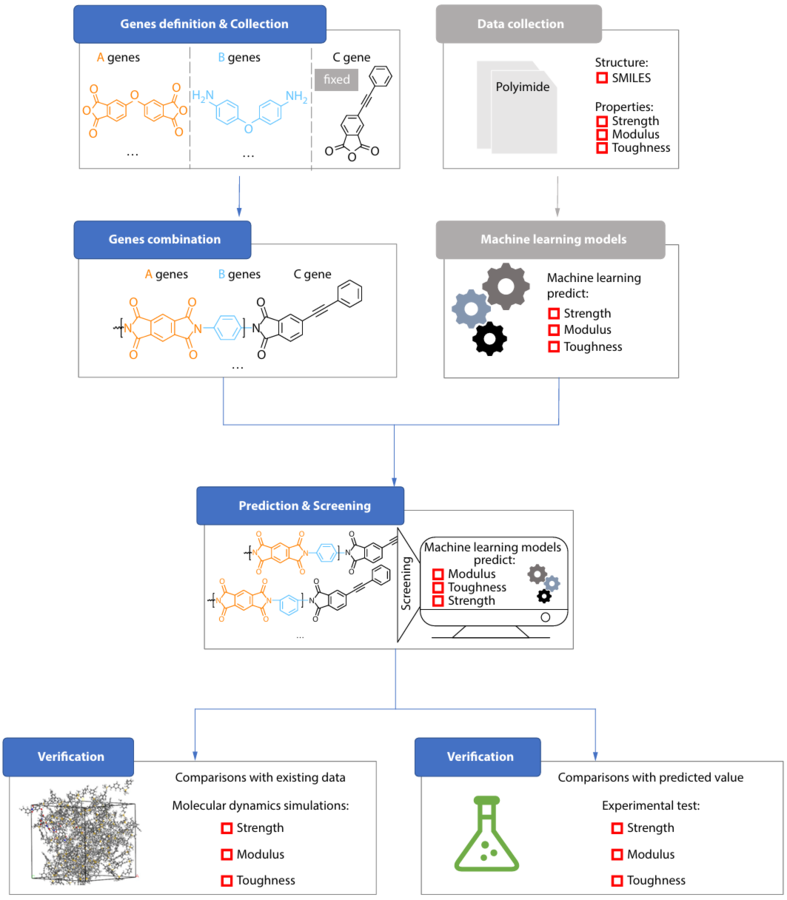
- The U.S. FDA approved STOBOCLO® (denosumab-bmwo) and OSENVELT® (denosumab-bmwo) as interchangeable with the reference products PROLIA® (denosumab) and XGEVA® (denosumab), respectively, for all approved indications, effective as of October 29, 2025
- The interchangeability (IC) designations of STOBOCLO and OSENVELT were granted based not only on the comparative clinical data – including pharmacokinetics, efficacy, safety and an immunogenicity study in postmenopausal women with osteoporosis[1] – but also on analytical data demonstrating similarity of STOBOCLO and OSENVELT versus the reference product
- The IC designations enhance patient access and provider choice in the treatment of osteoporosis-related fracture as well as cancer-related bone loss
INCHEON, South Korea, Oct. 30, 2025 /PRNewswire/ — Celltrion, Inc. today announced that the U.S. Food and Drug Administration (FDA) has designated STOBOCLO® (denosumab-bmwo) and OSENVELT® (denosumab-bmwo) as interchangeable biosimilars to the reference products PROLIA® (denosumab) and XGEVA® (denosumab), respectively, for all approved indications.
The interchangeability (IC) designation is a regulatory designation granted by the FDA, which means STOBOCLO and OSENVELT may now be substituted at the pharmacy for the reference products without consulting the prescriber, subject to state laws.[2]
“Today’s IC designations reinforce confidence in STOBOCLO and OSENVELT among physicians and pharmacists, facilitating a more seamless switch from the reference products to our denosumab biosimilars,” said Thomas Nusbickel, Chief Commercial Officer at Celltrion USA. “Building on our strong heritage in biosimilars, Celltrion remains committed to offering more affordable and much-needed treatment options to patients living with skeletal diseases, creating greater potential to deliver savings to patients and the U.S. healthcare system.”
The interchangeability designations of STOBOCLO and OSENVELT were based on the comprehensive evidence including the clinical results from Phase III clinical trials in postmenopausal women with osteoporosis designed to evaluate the efficacy, pharmacodynamics (PD), pharmacokinetics (PK), safety and immunogenicity of denosumab biosimilar to its reference product. [1]
STOBOCLO and OSENVELT were introduced in the U.S. market in July 2025. STOBOCLO is currently available in 60 mg/mL injection and OSENVELT is offered in 120 mg/1.7 mL (70 mg/mL) injection.
According to recent FDA draft guidance, biosimilar applicants can request an interchangeability designation using existing data from their Biologics License Application (BLA). Previously, the FDA granted this status only to biosimilars that submitted multiple switch studies meeting additional data criteria.
About STOBOCLO® (denosumab-bmwo) [3]
STOBOCLO® (denosumab-bmwo) is a receptor activator of NF-κb ligand (RANKL) inhibitor referencing PROLIA® (denosumab). STOBOCLO 60 mg/mL injection is approved by the FDA based on comprehensive data and clinical evidence confirming the therapeutic equivalence to PROLIA. In the U.S., STOBOCLO is approved to treat postmenopausal women with osteoporosis at high risk for fracture, to increase bone mass in men with osteoporosis at high risk for fracture, to treat glucocorticoid-induced osteoporosis in men and women at high risk for fracture, to increase bone mass in men at high risk for fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer, and to increase bone mass in women at high risk for fracture receiving adjuvant aromatase inhibitor therapy for breast cancer.
INDICATIONS
STOBOCLO® (denosumab-bmwo) is a RANK ligand (RANKL) inhibitor indicated for treatment:
- of postmenopausal women with osteoporosis at high risk for fracture
- to increase bone mass in men with osteoporosis at high risk for fracture or in men at high risk for fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer
- of glucocorticoid-induced osteoporosis in men and women at high risk for fracture
- to increase bone mass in women at high risk for fracture receiving an adjuvant aromatase inhibitor therapy for breast cancer
IMPORTANT SAFETY INFORMATION
WARNING: SEVERE HYPOCALCEMIA IN PATIENTS WITH ADVANCED KIDNEY DISEASE
Patients with advanced chronic kidney disease, including those on dialysis, face a higher risk of severe hypocalcemia after denosumab administration, with reported cases leading to hospitalization, life-threatening events, and fatalities.
The presence of chronic kidney disease-mineral bone disorder (CKD-MBD) markedly increases the risk of hypocalcemia in these patients
Before starting STOBOCLO® (denosumab-bmwo) in advanced chronic kidney disease patients, assess for CKD-MBD. Treatment should be supervised by a healthcare provider experienced in diagnosing and managing CKD-MBD.
STOBOCLO is contraindicated in hypocalcemia, pregnant women, and in patients with known hypersensitivity to denosumab.
Severe Hypocalcemia: Ensure adequate calcium and vitamin D; monitor for severe hypocalcemia.
Drug Products with Same Active Ingredient: Do not use with other denosumab products.
Hypersensitivity : If an anaphylactic or other clinically significant allergic reaction occurs, initiate appropriate therapy and discontinue further use of STOBOCLO.
Osteonecrosis of the Jaw (ONJ): ONJ can occur in patients on STOBOCLO. Conduct oral exams before treatment; maintain oral hygiene; consider discontinuation of STOBOCLO if ONJ develops.
Atypical Subtrochanteric and Diaphyseal Femoral Fractures: Monitor for thigh, hip, or groin pain; evaluate for fractures. Interruption of STOBOCLO therapy should be considered, pending a benefit-risk assessment, on an individual basis.
Multiple Vertebral Fractures (MVF) Following Discontinuation of Treatment: Increased risk post-discontinuation of denosumab; transition to alternative therapy if discontinuing STOBOCLO.
Serious Infections: Higher risk in denosumab users; assess benefit-risk profile, especially in immunocompromised patients. Assess the benefit-risk profile before starting STOBOCLO and reconsider its use if serious infections develop.
Dermatologic Adverse Reactions: Consider discontinuing STOBOCLO if severe dermatitis, eczema, or rashes occur.
Musculoskeletal Pain: Consider discontinuation of STOBOCLO if severe pain develops.
Bone Turnover Suppression: In clinical trials in women with postmenopausal osteoporosis, denosumab significantly suppressed bone remodelling; patients should be monitored for these outcomes.
Hypercalcemia in Pediatrics Patients with Osteogenesis Imperfecta: Not for pediatric use; hypercalcemia reported in patients osteogenesis imperfecta treated with denosumab products.
Most common Adverse Reactions:
- In (>5%) of patients with: Postmenopausal osteoporosis were back pain, pain in extremity, hypercholesterolemia, musculoskeletal pain, and cystitis. Pancreatitis has been reported in clinical trials. Male osteoporosis were back pain, arthralgia, and nasopharyngitis.
- Glucocorticoid-induced osteoporosis (> 3%) were back pain, hypertension, bronchitis, and headache.
- Bone loss due to hormone ablation for cancer (≥ 10%) were arthralgia and back pain. Pain in extremity and musculoskeletal pain have also been reported in clinical trials.
For more information, see Full Prescribing Information including Boxed Warning .
To learn more about the STOBOCLO REMs program please visit stoboclorems.com .
About OSENVELT® (denosumab-bmwo)[4]
OSENVELT® (denosumab-bmwo) is a receptor activator of NF-κb ligand (RANKL) inhibitor referencing XGEVA® (denosumab). OSENVELT 120 mg/1.7 mL (70 mg/mL) injection is approved by the FDA based on a robust clinical trial and comprehensive data confirming the therapeutic equivalence to XGEVA. In the U.S., OSENVELT is indicated to prevent skeletal-related events in patients with multiple myeloma and in patients with bone metastases from solid tumors, to treat adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity, and to treat hypercalcemia of malignancy refractory to bisphosphonate therapy.
INDICATION
OSENVELT® (denosumab-bmwo) is indicated for:
- Prevention of skeletal-related events in patients with multiple myeloma and in patients with bone metastases from solid tumors.
- Treatment of adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity.
- Treatment of hypercalcemia of malignancy refractory to bisphosphonate therapy.
IMPORTANT SAFETY INFORMATION
Contraindications: Patients with hypocalcemia or with known clinically significant hypersensitivity to denosumab products.
Drug Products with Same Active Ingredient. Patients receiving OSENVELT should not receive other denosumab products concomitantly.
Hypersensitivity. If an anaphylactic or other clinically significant allergic reaction occurs, initiate appropriate therapy and discontinue further use of OSENVELT.
Hypocalcemia. Severe hypocalcemia can occur, and fatal cases have been reported. Monitor calcium levels and calcium and vitamin D intake.
Osteonecrosis of the Jaw (ONJ): ONJ can occur in patients on OSENVELT. Conduct oral exams and appropriate preventive dentistry before and during treatment; maintain oral hygiene and avoid invasive dental procedures; consider discontinuation of OSENVELT if ONJ develops.
Atypical Subtrochanteric and Diaphyseal Femoral Fractures: Monitor for thigh, hip, or groin pain; evaluate for fractures. Interruption of OSENVELT therapy should be considered, pending a benefit-risk assessment, on an individual basis.
Hypercalcemia Following Treatment Discontinuation in Patients with Giant Cell Tumor of Bone and in Patients with Growing Skeletons. Clinically significant hypercalcemia, potentially requiring hospitalization, can occur within a year after stopping denosumab in patients with giant cell tumor of bone or growing skeletons; monitor serum calcium and manage calcium and vitamin D needs post-discontinuation.
Multiple Vertebral Fractures (MVF) Following Treatment Discontinuation. Increased risk post-discontinuation of denosumab; evaluate for risk for vertebral fractures after discontinuing OSENVELT.
Embryo-Fetal Toxicity. Denosumab may cause fetal harm; verify pregnancy status before starting OSENVELT and advise effective contraception during treatment and for 5 months after the last dose.
Most common Adverse Reactions:
- Bone Metastasis from Solid Tumors (≥ 25%) were fatigue/asthenia, hypophosphatemia, and nausea.
- In patients (≥ 10%) with: Multiple Myeloma were diarrhea, nausea, anemia, back pain, thrombocytopenia, peripheral edema, hypocalcemia, upper respiratory tract infection, rash, and headache; Giant Cell Tumor of Bone were arthralgia, headache, nausea, back pain, fatigue, and pain in extremity.
- Hypercalcemia of Malignancy (> 20%) were nausea, dyspnea, decreased appetite, headache, peripheral edema, vomiting, anemia, constipation, and diarrhea.
For more information, see Full Prescribing Information .
About Celltrion , Inc.
Celltrion is a leading biopharmaceutical company that specializes in researching, developing, manufacturing, marketing and sales of innovative therapeutics that improve people’s lives worldwide. Celltrion is a pioneer in the biosimilar space, having launched the world’s first monoclonal antibody biosimilar. Our global pharmaceutical portfolio addresses a range of therapeutic areas including immunology, oncology, hematology, ophthalmology and endocrinology. Beyond biosimilar products, we are committed to advancing our pipeline with novel drugs to push the boundaries of scientific innovation and deliver quality medicines. For more information, please visit our website www.celltrion.com/en-us. and stay updated with our latest news and events on our social media – LinkedIn, Instagram, X, and Facebook.
About Celltrion USA
Celltrion USA is Celltrion’s U.S. subsidiary established in 2018. Headquartered in New Jersey, Celltrion USA is committed to expanding access to innovative biologics to improve care for U.S. patients. Celltrion’s FDA-approved biosimilar products in immunology, oncology, hematology, and endocrinology include: INFLECTRA® (infliximab-dyyb), TRUXIMA® (rituximab-abbs), HERZUMA® (trastuzumab-pkrb), VEGZELMA® (bevacizumab-adcd), YUFLYMA®(adalimumab-aaty), AVTOZMA® (tocilizumab-anho), STEQEYMA® (Ustekinumab-stba) STOBOCLO® (denosumab-bmwo), OSENVELT® (denosumab-bmwo), OMLYCLO® (omalizumab-igec), and EYDENZELT® (aflibercept-boav) as well as the novel biologic ZYMFENTRA® (infliximab-dyyb). Celltrion USA will continue to leverage Celltrion’s unique heritage in biotechnology, supply chain excellence and best-in-class sales capabilities to improve access to high-quality biopharmaceuticals for U.S. patients. For more information, please visit www.celltrionusa.com. and stay updated with our latest news and events on our social media – LinkedIn
FORWARD-LOOKING STATEMENT
Certain information set forth in this press release contains statements related to our future business and financial performance and future events or developments involving Celltrion, Inc. and its subsidiaries that may constitute forward-looking statements, under pertinent securities laws.
These statements may be also identified by words such as “prepares”, “hopes to”, “upcoming”, “plans to”, “aims to”, “to be launched”, “is preparing”, “once gained”, “could”, “with the aim of”, “may”, “once identified”, “will”, “working towards”, “is due”, “become available”, “has potential to”, the negative of these words or such other variations thereon or comparable terminology.
In addition, our representatives may make oral forward-looking statements. Such statements are based on the current expectations and certain assumptions of Celltrion, Inc. and its subsidiaries’ management, of which many are beyond its control.
Forward-looking statements are provided to allow potential investors the opportunity to understand management’s beliefs and opinions in respect to the future so that they may use such beliefs and opinions as one factor in evaluating an investment. These statements are not guarantees of future performance and undue reliance should not be placed on them.
Such forward-looking statements necessarily involve known and unknown risks and uncertainties associated with the company’s business, including the risk factors disclosed in its Annual Report and/or Quarterly Reports, which may cause actual performance and financial results in future periods to differ materially from any projections of future performance or results expressed or implied by such statements.
Celltrion, Inc. and its subsidiaries undertake no obligation to update forward-looking statements if circumstances or management’s estimates or opinions should change except as required by applicable securities laws.
Trademarks
STOBOCLO® and OSENVELT® are registered trademarks of Celltrion, Inc.
PROLIA® and XGEVA® are registered trademarks of Amgen Inc.
References
US-CT-P41-25-00008
For further information please contact:
Katie Gallagher
[email protected]
+1 617-657-1324
SOURCE Celltrion