- Plus Leuprolide Reduced Risk of Death by 40% vs Leuprolide Alone in Men with a Type of Advanced Prostate Cancer Business Wire
- Scientists identify drug combination that can weaken prostate cancer cells The Independent
- Block enzymes tied to the…
Blog
-
Plus Leuprolide Reduced Risk of Death by 40% vs Leuprolide Alone in Men with a Type of Advanced Prostate Cancer – Business Wire
-
Dark matter is not still, it behaves like a cosmic superfluid: Study
It may sound unbelievable, but new research suggests that instead of being featureless, dark matter could actually behave like a cosmic superfluid, forming swirling vortex lines and stable rotating cores known as solitons inside…
Continue Reading
-

Game-Tying Drive Comes Up Short at Stanford
PALO ALTO, Calif. – The Florida State football team lost at Stanford 20-13 on Saturday night at Stanford Stadium in Palo Alto, California.
With 63 seconds remaining, the Seminoles (3-4, 0-4 ACC) drove down the field 79 yards to try and tie…Continue Reading
-

FLAURA2 Trial: Osimertinib Plus Chemotherapy Improves Overall Survival Across Prognostic Subgroups in EGFR-Mutated Advanced NSCLC — Presented at ESMO 2025
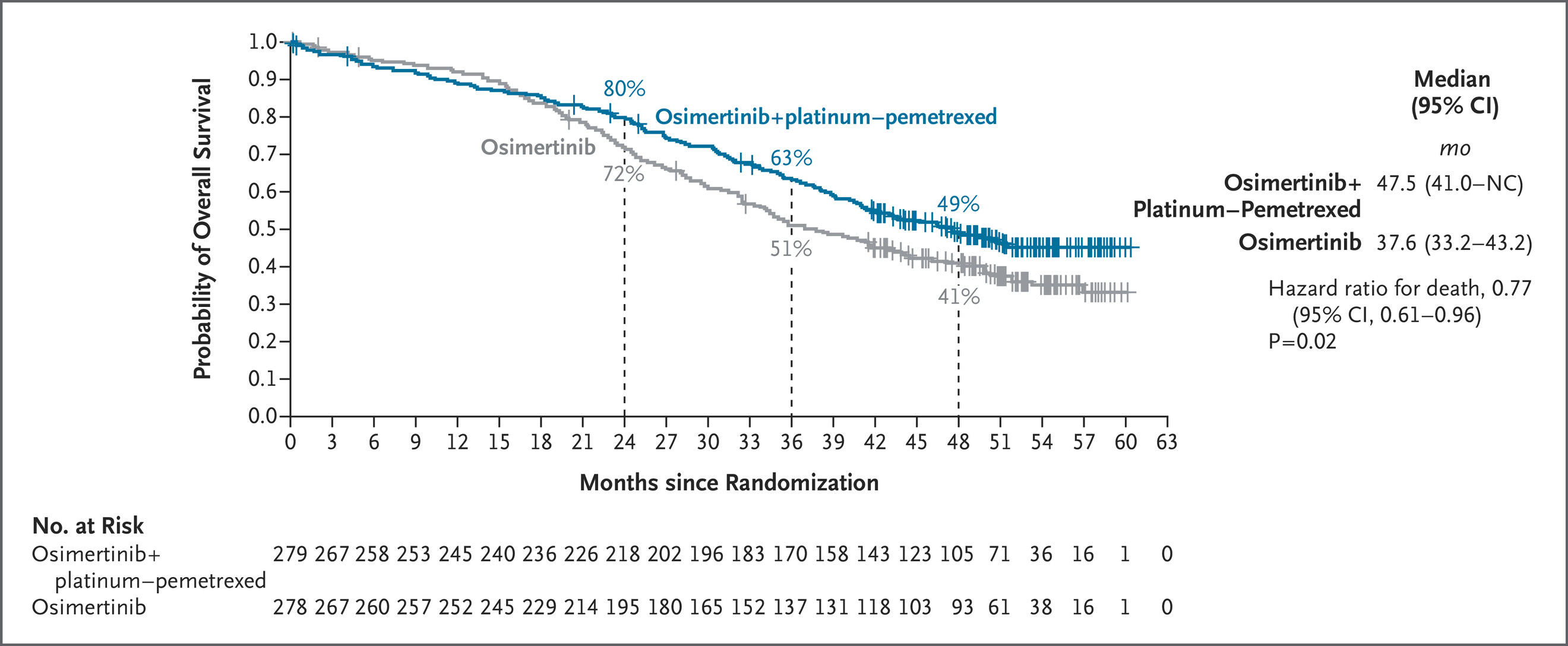
The Phase 3 FLAURA2 trial (NCT04035486) continues to strengthen the role of combination therapy in the first-line management of EGFR-mutated (EGFRm) advanced non–small cell lung cancer (NSCLC). At ESMO 2025, investigators reported the final overall survival (OS) analysis, showing that osimertinib (osi) plus platinum–pemetrexed chemotherapy (CTx) achieved a statistically significant and clinically meaningful OS improvement compared with osimertinib monotherapy.
This milestone confirms the combination as a first-line standard of care in EGFRm NSCLC, offering durable efficacy across key subgroups, including those with traditionally poor prognostic features such as CNS metastases, L858R mutations, plasma-detectable EGFRm, and altered TP53.
Methods
The FLAURA2 trial enrolled 557 patients with EGFRm advanced NSCLC, who were randomized 1:1 to receive osimertinib plus chemotherapy (n=279) or osimertinib alone (n=278). The chemotherapy regimen consisted of platinum–pemetrexed, administered concurrently with osimertinib.
Baseline CNS imaging (CT or MRI) was mandatory to identify central nervous system metastases. EGFR mutation subtypes (Ex19del or L858R) were confirmed through central or locally approved assays. Plasma EGFR mutation detection was evaluated via droplet digital PCR (Biodesix), while TP53 mutation status was determined using FoundationOne CDx.
OS outcomes were analyzed using an unstratified Cox proportional hazards model, with subgroup analyses conducted based on baseline prognostic factors.
Results
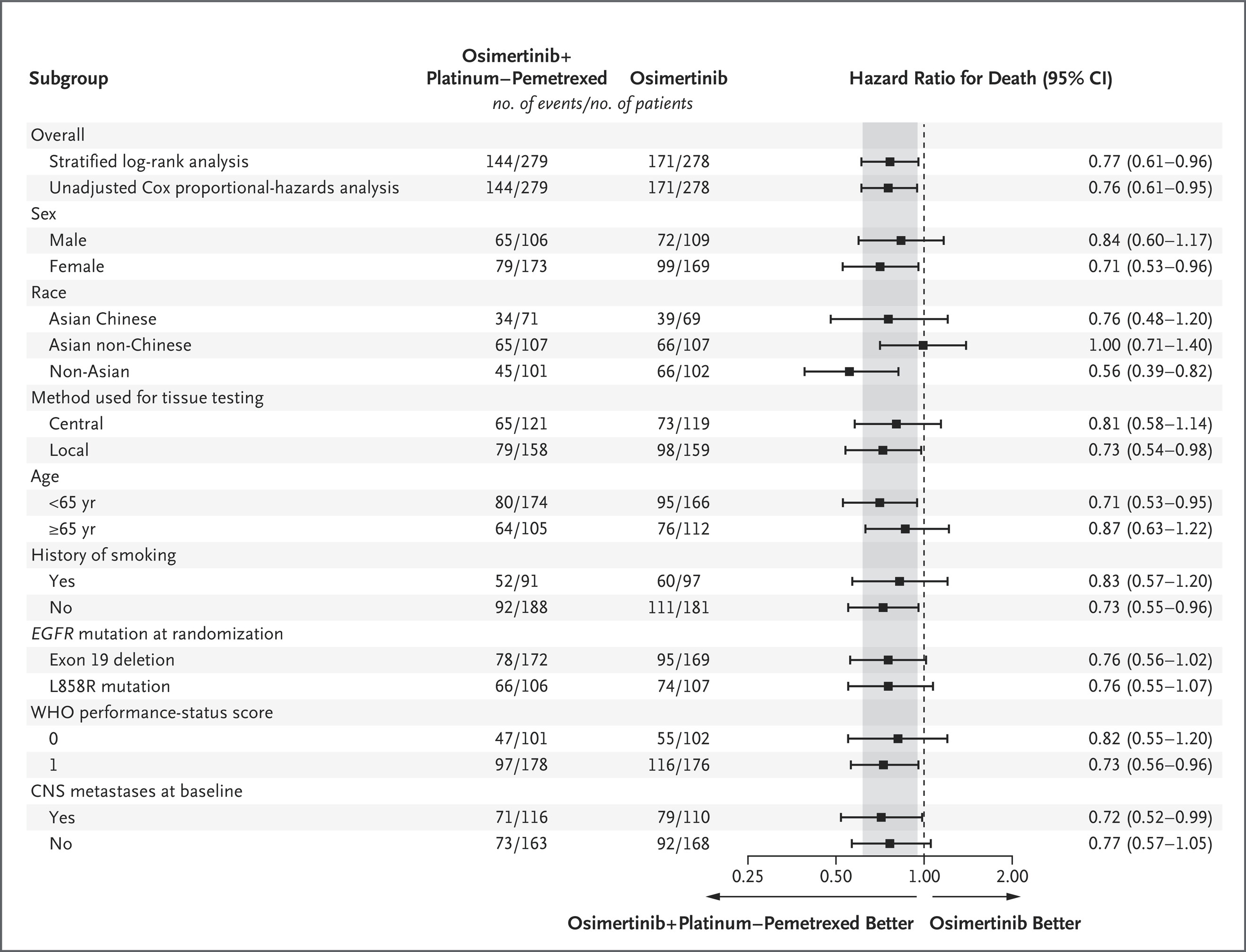
A total of 557 patients were included: 279 in the osimertinib + CTx arm and 278 in the osimertinib monotherapy arm. The overall hazard ratio (HR) for OS was 0.77 (95% CI 0.61–0.96; p=0.02), corresponding to a 23% reduction in risk of death with the combination regimen.
CNS Metastases
In patients with baseline CNS metastases, median OS reached 40.9 months (95% CI 35.2–46.6) with osimertinib + CTx versus 29.7 months (95% CI 25.6–35.8) with monotherapy (HR 0.72; 95% CI 0.52–0.99).
EGFR Mutation Subtype
Both EGFRm subgroups derived benefit.
- L858R: median OS 38.1 vs 32.4 months (HR 0.76; 95% CI 0.55–1.07)
- Ex19del: median OS not reached (NR) vs 43.0 months (HR 0.76; 95% CI 0.56–1.02)
Plasma EGFR Mutation Detection
Patients with detectable plasma EGFRm achieved median OS 38.4 vs 32.5 months (HR 0.79; 95% CI 0.60–1.03), while those with undetectable mutations had not reached median OS in either arm.
TP53 Alteration Status
Among patients with altered TP53, OS improved to 51.1 months vs 43.1 months (HR 0.71; 95% CI 0.40–1.27), showing consistent benefit across molecular profiles.
No new safety signals were reported, confirming that the addition of chemotherapy did not compromise the established tolerability of osimertinib.
Interpretation
The survival improvement observed with osimertinib plus chemotherapy extended across every prognostic subgroup examined. The HRs for OS within each category closely mirrored those of the overall intent-to-treat (ITT) population, highlighting the robustness and reproducibility of the benefit.
Particularly noteworthy were the gains among patients with CNS metastases and L858R mutation, two subsets traditionally associated with poorer prognosis. These data underline the capacity of the combination regimen to overcome key resistance mechanisms and delay disease progression.
Long-Term Follow-Up and Analytical Considerations
The long-term follow-up from the FLAURA2 trial at ESMO 2025 provides the most comprehensive survival dataset to date for EGFR-mutated NSCLC treated with first-line osimertinib plus chemotherapy. With several subgroups not yet reaching median OS, the sustained separation of survival curves indicates a persistent and cumulative treatment effect extending well beyond the chemotherapy phase.
Investigators emphasized that the survival curves for osimertinib + CTx began to diverge early—within the first year of treatment—and remained separated throughout follow-up, suggesting that early dual blockade of EGFR signaling and microenvironmental suppression via chemotherapy offers durable control of micrometastatic disease.
The analysis also highlights that median OS was not reached in multiple subgroups, demonstrating an ongoing trend toward improved long-term outcomes. The consistency of benefit across genomic and clinical subgroups—including those with TP53 alterations or detectable circulating tumor DNA—supports the biological rationale that concurrent chemotherapy may help prevent the emergence of resistant clones.
Methodologically, the trial’s unstratified Cox model ensured unbiased subgroup comparisons and confirmed that the treatment effect was independent of baseline risk characteristics. Furthermore, despite crossover and subsequent therapies available post-progression, the OS advantage with osimertinib plus chemotherapy persisted, reinforcing the value of front-line combination therapy over sequential monotherapy strategies.
No late-emerging toxicities or cumulative safety concerns were identified during long-term follow-up, strengthening the safety profile of the regimen. Together, these findings establish osimertinib plus chemotherapy as a durable, well-tolerated, and biologically rational first-line standard of care for patients with EGFR-mutated advanced NSCLC.
Read Full Abstract on ESMO Congress 2025 Website
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
Continue Reading
-
AOL is part of the Yahoo family of brands.
AOL is part of the Yahoo family of brands.
When you use our sites and apps, we use cookie policy.”>cookies to:-
…
Continue Reading
-
The Sky Today on Sunday, October 19: The Moon and Venus before dawn – Astronomy Magazine
- The Sky Today on Sunday, October 19: The Moon and Venus before dawn Astronomy Magazine
- Don’t Miss Sunday’s Beautiful Crescent Moon Beside Brilliant Venus Forbes
- When the Sky Smiles: A Rare Triple Conjunction of the Moon, Venus, and Regulus…
Continue Reading
-

Latest Belfast Giants News | Belfast Giants
First Period
It was the visitors who came out of the traps quickest and made Jackson Whistle work. Vlastimil Dostalek rattled the crossbar before Whistle made a good blocker save from Jaka Sturm. Momentum would swing back towards the…
Continue Reading
-

Delete Any Texts On Your Phone If You See This Google Warning
Delete all these texts.
getty
Text attacks on Android and iPhone owners in America, Europe and elsewhere are totally out of control. This billion-dollar industry now defrauds millions of smartphone owners. The malicious technologies that execute…
Continue Reading
-

EV + Pembrolizumab Boosts Survival in MIBC
KEYNOTE-905/EV-303 (NCT03924895), a phase 3, open-label, randomized trial, evaluated perioperative enfortumab vedotin (EV) plus pembrolizumab (pembro) compared with surgery alone in patients with muscle-invasive bladder cancer (MIBC) who were ineligible for or declined cisplatin-based chemotherapy.
Presented by Prof. Christof Vulsteke at the ESMO Congress 2025, the study demonstrated that adding EV + pembro to radical cystectomy with pelvic lymph node dissection resulted in significant improvements in event-free survival (EFS), overall survival (OS), and pathologic complete response (pCR). These findings mark a major advance for cisplatin-ineligible MIBC, establishing a potential new perioperative standard of care.
Background
Radical cystectomy with pelvic lymph node dissection (RC + PLND) remains the standard of care for patients with muscle-invasive bladder cancer. However, nearly half of these patients are ineligible for cisplatin-based chemotherapy due to renal dysfunction, frailty, or comorbidities, leaving a substantial population without effective perioperative options.
Previous studies have shown limited benefit from surgery alone in this setting, underscoring the unmet need for alternative strategies. Enfortumab vedotin, an antibody–drug conjugate targeting Nectin-4, combined with pembrolizumab, has demonstrated potent antitumor activity and a favorable safety profile in metastatic urothelial cancer, providing a strong rationale for evaluation in the perioperative context.
Methods
KEYNOTE-905/EV-303 is a randomized, open-label, phase 3 trial that enrolled adults with MIBC (T2–T4aN0M0 or T1–T4aN1M0) who were cisplatin-ineligible per Galsky criteria or declined cisplatin.
Participants were randomized 1:1 to receive:- EV + pembro arm: Three cycles of EV (1.25 mg/kg on days 1 and 8) plus pembrolizumab (200 mg every three weeks) before RC + PLND, followed by six additional EV cycles and 14 cycles of pembrolizumab postoperatively.
- Control arm: RC + PLND alone, with adjuvant nivolumab permitted when clinically indicated.
The primary endpoint was event-free survival (EFS) by blinded independent central review (BICR).Key secondary endpoints included overall survival (OS), pathologic complete response (pCR), and safety.
Timeline of study development:
- The study initiated in 2019 with two treatment arms—perioperative pembrolizumab + RC + PLND vs RC + PLND alone—randomized 1:1.
- In 2020, a third arm was added, introducing perioperative EV + pembro + RC + PLND, changing the randomization to 1:1:1.
- By 2022, the design evolved: the pembrolizumab-alone arm was discontinued, and randomization was updated to 1:1 between EV + pembro + RC + PLND (EV + pembro arm) and RC + PLND (control arm).
- The inclusion criteria were expanded to include patients who were cisplatin-eligible but declined cisplatin, broadening the real-world relevance of the study population.
- Adjuvant nivolumab was permitted in the control arm per local guidelines.
Results
A total of 344 participants were randomized between December 2020 and June 2024 (170 to EV + pembro; 174 to control). Over 80% were cisplatin-ineligible, and the median follow-up was 25.6 months (range 11.8–53.7).The combination regimen produced statistically significant and clinically meaningful improvements across all efficacy endpoints:
- Event-Free Survival (EFS): Median EFS was not reached with EV + pembro versus 15.7 months with control (HR 0.40; 95% CI 0.28–0.57; P<0.0001). At one years, EFS rates were 77.8% vs 55.1%, respectively and at two years, 74,7% vs 39,4%
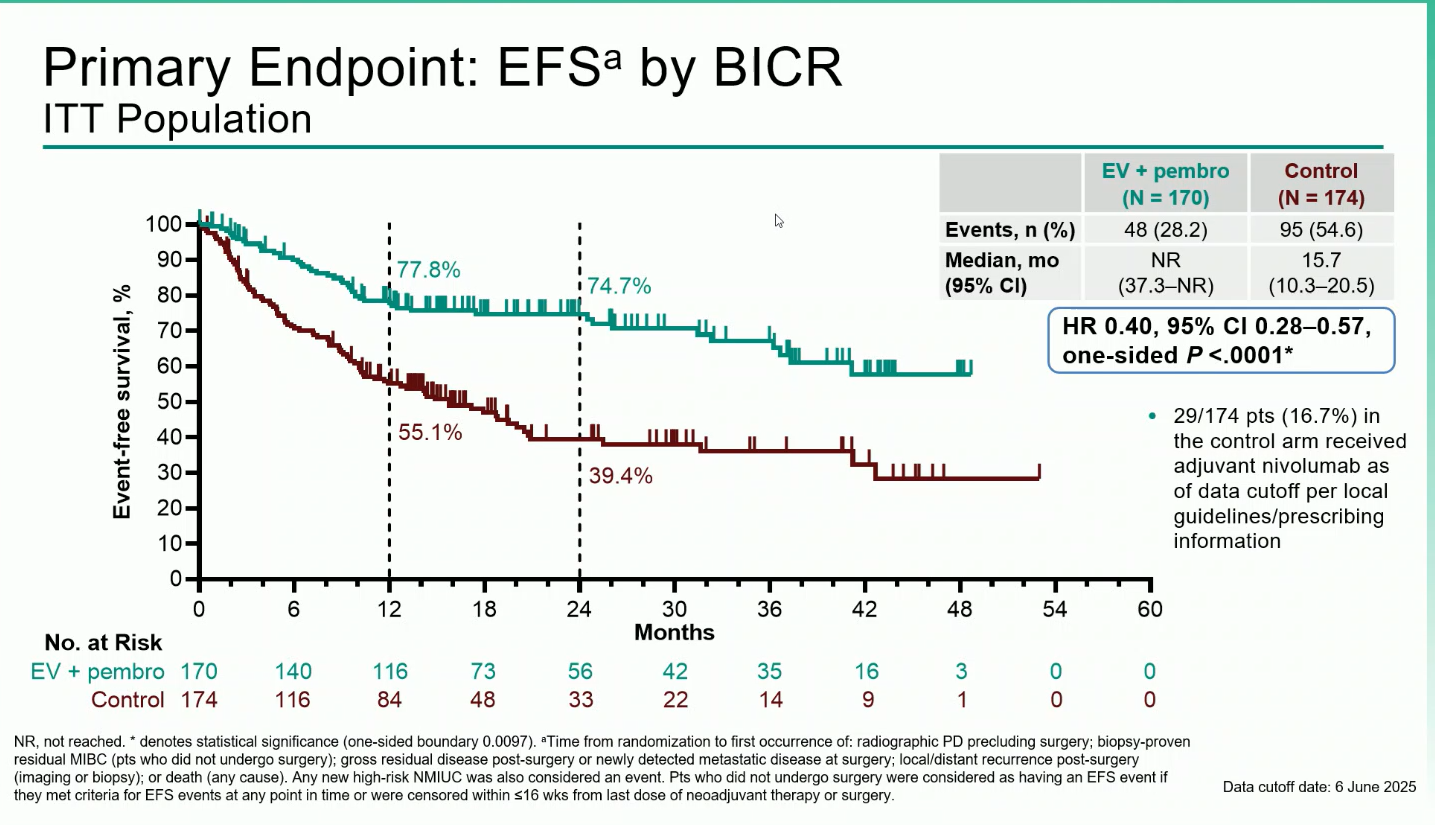
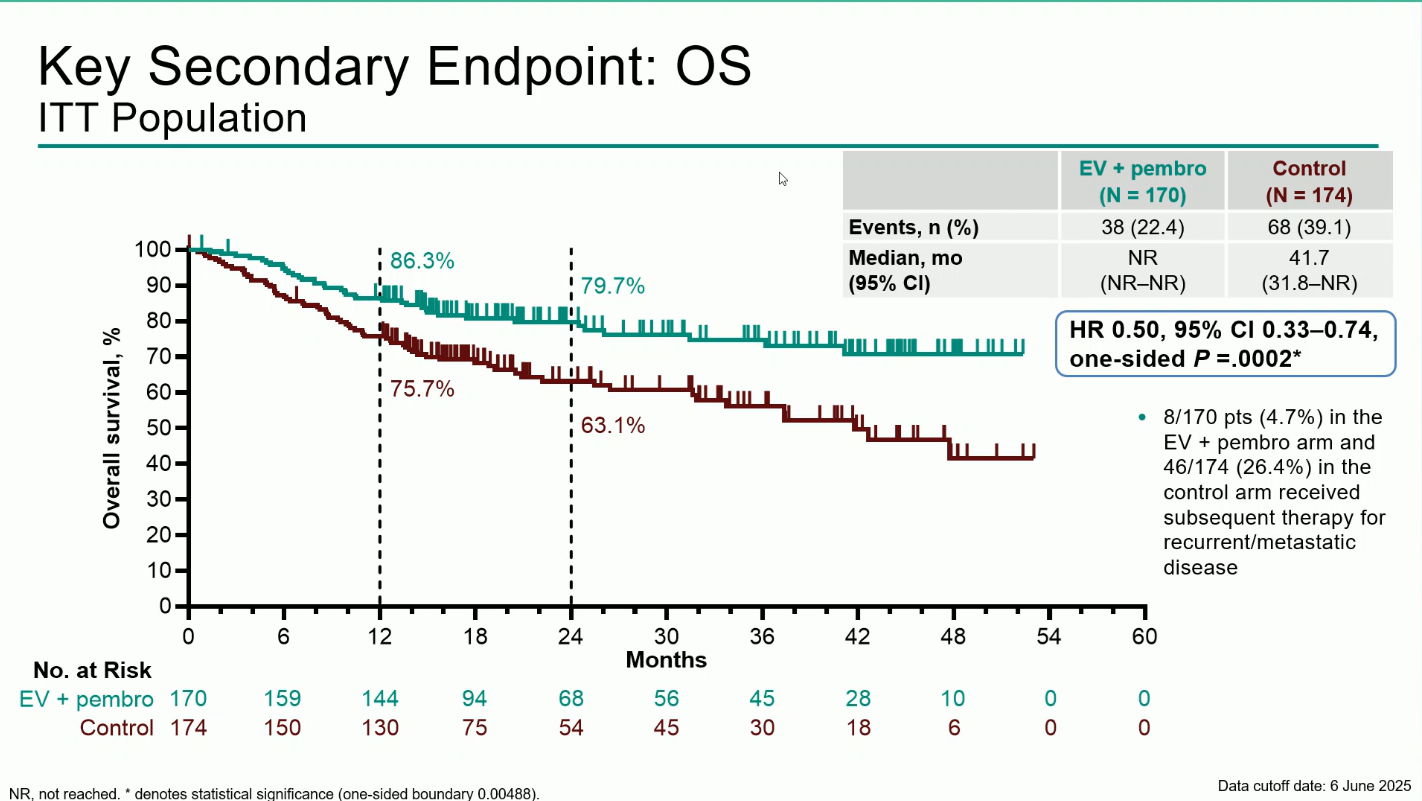
- Overall Survival (OS): Median OS was not reached with EV + pembro versus 41.7 months with control (HR 0.50; 95% CI 0.33–0.74; P=0.0002). The 12-month OS rates were 86.3% vs 75.7%, and the 24-months OS rates were 79,7% vs 63,1%
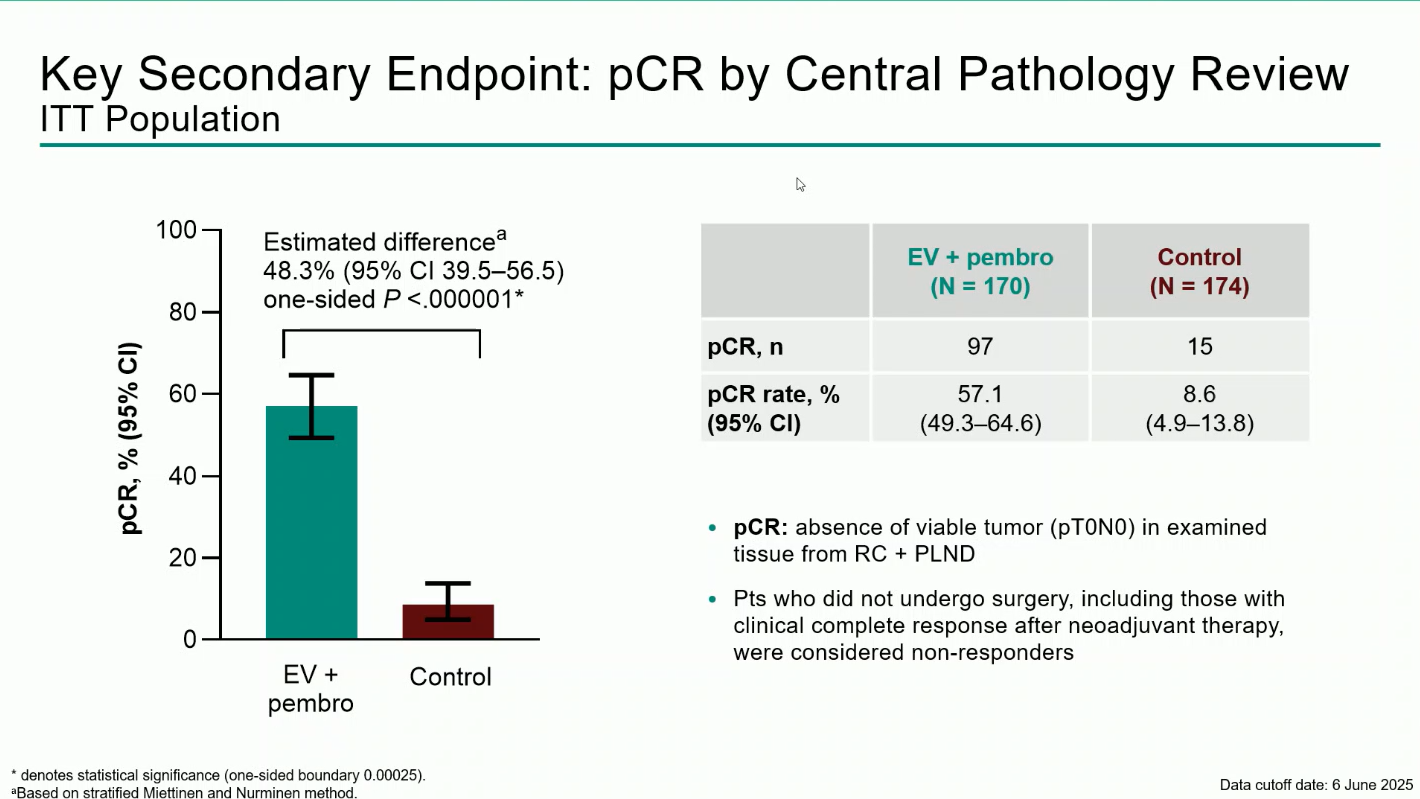
- Pathologic Complete Response (pCR): 57.1% with EV + pembro vs 8.6% with control, an absolute difference of 48.3% (95% CI 39.5–56.5; P<0.0001).
Safety:
Treatment-emergent adverse events (TEAEs) occurred in nearly all patients (100% in EV + pembro vs 64.8% in control). Grade ≥3 events were reported in 71.3% and 45.9%, respectively. The most frequent grade ≥3 adverse events of special interest were severe skin reactions (11.4%) related to pembrolizumab and cutaneous toxicity (10.8%) associated with enfortumab vedotin.
Despite the high incidence of AEs, the safety profile remained manageable and consistent with prior studies, and no new safety signals were identified.
Conclusions
The KEYNOTE-905/EV-303 trial demonstrated that adding perioperative enfortumab vedotin plus pembrolizumab to standard surgery significantly improved event-free survival, overall survival, and pathologic complete response rates in patients with cisplatin-ineligible MIBC.
These findings establish EV + pembrolizumab as the first perioperative regimen to improve outcomes versus RC + PLND alone in this population, offering a potential new standard of care.
You can read the full abstract here.
Continue Reading
-
'No Place Is Risk-Free Until The World Is Polio-Free:' Bill Gates Urges Global Action To Close Deadly Immunity Gaps – Benzinga
- ‘No Place Is Risk-Free Until The World Is Polio-Free:’ Bill Gates Urges Global Action To Close Deadly Immunity Gaps Benzinga
- Salisbury Rotary Club to plant 4,000 purple crocus corms to mark World Polio Day Yahoo News UK
- Experts emphasise…
Continue Reading