Participants
This was a prospective observational cohort study conducted at Hospital Likas, Kota Kinabalu, Sabah, East Malaysia. The study was approved by the Medical Research Sub-Committee of the Malaysian Ministry of Health (Reference…
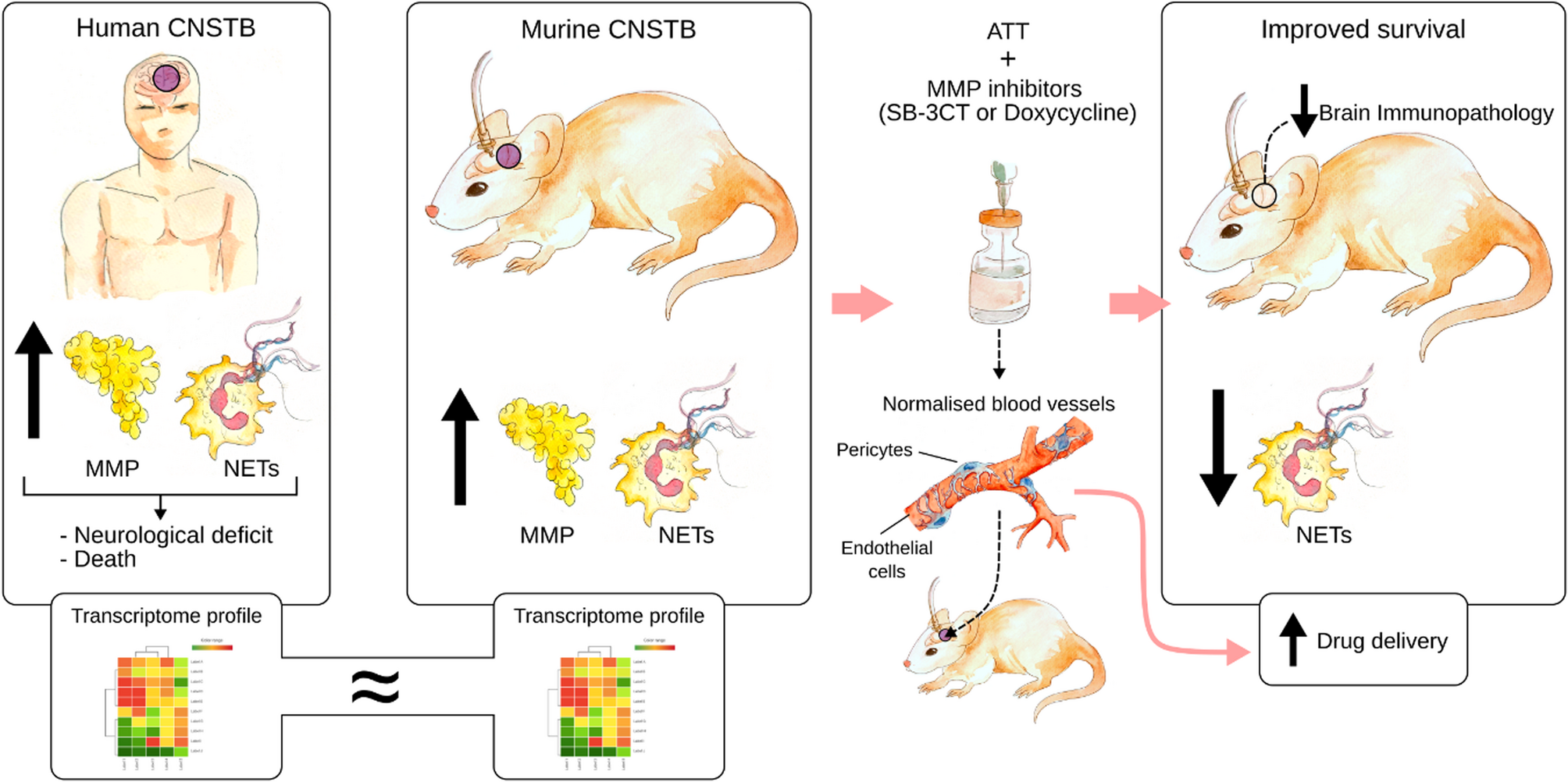
This was a prospective observational cohort study conducted at Hospital Likas, Kota Kinabalu, Sabah, East Malaysia. The study was approved by the Medical Research Sub-Committee of the Malaysian Ministry of Health (Reference…

Switch 2 is Nintendo’s first major console that simply adds a “2” to the name of its predecessor. So naturally, it makes sense to compare the two – and today, we’re looking at the launch of the original Nintendo Switch in 2017 and the…

Plasmacytoma is a rare neoplastic disorder caused by the abnormal monoclonal proliferation of mature B cells, accounting for approximately 10% of all hematological tumors.1 Plasmacytomas are clinically and pathologically classified into three types: multiple myeloma (MM), solitary bone plasmacytoma (SBP), and solitary extramedullary plasmacytoma (SEP).2 SEP is an extremely rare soft tissue mass composed of monoclonal plasma cells, accounting for 3–4% of all plasmacytomas. Nearly 80% of SEPs are located in the head and neck, particularly in the upper respiratory tract, followed by the gastrointestinal tract. SEPs rarely occur in the lungs, thyroid, breasts, testes, skin, or at other sites.3 Due to its rarity, SEP in the sternoclavicular joint has not been thoroughly studied. In this case report, we describe a 62-year-old male patient who presented with sternoclavicular joint pain and swelling. After excluding infectious and rheumatologic disorders, the patient was ultimately diagnosed with SEP.
A 62-year-old male patient presented with progressively worsening pain and swelling in the left sternoclavicular joint for two months without any apparent cause. The patient visited the orthopedic department and was diagnosed with “sternoclavicular joint inflammation”. He received oral and topical analgesics as well as intravenous anti-inflammatory infusions, but his condition did not improve. Additionally, he experienced pain in the left neck and shoulder areas and had limited mobility. He underwent chest magnetic resonance imaging (MRI) with diffusion-weighted imaging (DWI) in the orthopedic department, which led to his admission for treatment at the rheumatology department of the same hospital. After admission, the patient experienced pain and swelling in the sternoclavicular joint as well as pain in the left neck and shoulder with limited mobility. The patient had hypertension for more than 20 years and was currently not receiving antihypertensive treatment. He reported that his blood pressure was well-controlled. He also had diabetes for over 15 years and was currently taking empagliflozin for glycemic control with satisfactory results.
The patient’s temperature was 36.8 °C, respiratory rate was 20 breaths/min, pulse rate was 84 beats/min, and blood pressure was 96/52 mmHg. His general condition was fair, and there was no enlargement of the superficial lymph nodes throughout the body. He was conscious, and physical examination of the heart, lungs, and abdomen revealed no positive signs. He exhibited redness and swelling of the left sternoclavicular joint, accompanied by significant tenderness and increased skin temperature. He also experienced pain in the left neck and shoulder and had limited mobility. There was no swelling or tenderness in the other joints and no rashes on the palms or soles of his feet.
Laboratory tests revealed a complete blood count, inflammatory markers, biochemical profile, thyroid function, and partial hematological parameters (Table 1). The patient tested positive for hepatitis B virus e antibody and core antibody but negative for anti-thyroglobulin antibody, anti-thyroid peroxidase antibody, HBV DNA, tuberculosis infection T-cell assay (T-SPOT.TB), hepatitis C virus antibody, anti-Treponema pallidum antibody, HIV antigen/antibody, and HLA-B27. Coagulation function, antinuclear antibody profile, tumor markers, anti-neutrophil cytoplasmic antibodies (ANCA), rheumatoid immunological markers, anti-cardiolipin antibodies, immunoglobulins, and complements were all within normal limits. No monoclonal immunoglobulins were detected in serum or urine samples.
|
Table 1 Laboratory Results of First Admission
|
A chest MRI T2-weighted imaging (T2WI) sequence showed abnormal signals at the sternal end of the left clavicle, with adjacent soft tissue edema (Figure 1). Ultrasonography of the sternoclavicular joint indicated bilateral sternoclavicular joint inflammation with irregular bone cortices. Whole-body bone ECT showed no typical signs of bone metastasis. Pelvic CT scan without contrast showed no evidence of sacroiliitis. Based on the patient’s negative HLA-B27 status, ankylosing spondylitis (AS) was tentatively excluded.
 |
Figure 1 Chest MRI T2WI sequence showed abnormal signals at the sternal end of the left clavicle with adjacent soft tissue edema.
|
Blood Next-Generation Sequencing (NGS) indicated the presence of Pseudomonas, with a sequence count of 599151 and a relative abundance of 99.97%. It also identified the Pseudomonas aeruginosa complex with a sequence count of 598213 and a confidence level of 99%. Soft tissue culture and drug sensitivity tests of the sternoclavicular joint revealed the presence of Pseudomonas aeruginosa (50%) and Proteus mirabilis (50%), both of which were sensitive to meropenem. The results of the Brucella antibody test were negative.
The patient successively received pain-relief treatment with non-steroid anti-inflammatory drugs such as etoricoxib tablets, and tramadol sustained-release tablets. The patient also received a single intramuscular injection of 1 mL of compound betamethasone and an intravenous infusion of 40 mg methylprednisolone once daily for three days. Based on the result of culture, infection was considered, and the patient was administered oral linezolid tablets (0.6 g twice daily for two days) and an intravenous infusion of meropenem (0.5 g trice daily for three days, followed by 1 g trice daily for eight days) for anti-infection treatment. Despite therapeutic interventions, the patient’s joint pain persisted with unremarkable changes in inflammatory markers (Table 2), necessitating further diagnostic workup.
 |
Table 2 Laboratory Results Before Transfer to Hematology Department
|
As malignancy could not be excluded, a biopsy of the left sternoclavicular joint was performed. Pathological examination revealed an abundant infiltration of plasma cells and neutrophils within the fibrous tissue, with some plasma cells exhibiting atypia and light-chain restriction. Plasmacytoma remains a diagnostic consideration, prompting the recommendation for repeat extensive biopsies to confirm the diagnosis. The patient was subsequently transferred to the Hematology Department for further management.
The patient underwent positron emission tomography (PET)-CT in the hematology department. The results indicated swelling of the soft tissue around the left sternoclavicular joint, roughness of the joint surface, and increased fluorodeoxyglucose metabolism. Reactive hyperplasia of the lymph nodes in the mediastinum, pulmonary hilum, and porta hepatis was considered. The increased uptake in some parts of the colon, rectum, and small intestine is thought to be due to inflammation or physiological reasons. No other significant abnormalities were detected (Figure 2).
 |
Figure 2 PET-CT indicates soft tissue swelling around the left sternoclavicular joint with increased FDG uptake.
|
The bone marrow routine test showed that plasma cells accounted for 4%, with some morphological changes. The number of megakaryocytes increased slightly and some showed hyperfunctioning with platelet production. Flow cytometry analysis did not reveal any significant abnormalities. A bone marrow biopsy indicated active bone marrow proliferation (Figure 3).
 |
Figure 3 Bone marrow routine examination indicates that plasma cells account for 4%.
|
Because of insufficient tissue in the initial biopsy specimen, a repeat biopsy (left sternoclavicular joint biopsy) was performed on August 28, 2024. The pathological findings revealed fibrous tissue with scattered lymphocytes and plasma cell infiltration. Neoplastic lesions could not be excluded, and excisional biopsy was recommended for a definitive diagnosis; therefore, the patient was transferred to thoracic surgery for thoracoscopic thymectomy and mediastinal lesion resection. During the surgery, significant adhesions were observed in the left pleural cavity. A solid mass measuring 4×5 cm with a nodular appearance was found at the sternoclavicular joint of the left pleura. Hypertrophy of the left thymus was observed. Pathological examination of the mediastinal mass and thymus revealed fibro-fatty tissue with focal infiltration of a few plasma cells and light chain restriction. Plasma cell-derived tumors cannot be ruled out. Immunohistochemistry revealed CD56 (-), CD138 (+), CD38 (+), and Kappa ISH (++), indicating light chain restriction, and Lambda ISH (+).
Based on the histopathological findings, routine bone marrow tests, immunohistochemistry (IHC), PET-CT, and other diagnostic evaluations, the patient met the diagnostic criteria for SEP.4 Following local radiotherapy, pain in the left sternoclavicular joint was significantly alleviated after treatment completion. After nine months of follow-up, the patient’s joint pain has completely resolved. Laboratory findings were unremarkable (Table 3), and a repeat chest CT showed no evidence of local recurrence or disease progression.The timeline of the case was illustrated in Figure 4.
 |
Table 3 Laboratory Results at Nine-months Follow-up
|
 |
Figure 4 Timeline of the case report.
|
Plasma cell neoplasms are malignant neoplasms characterized by monoclonal proliferation of terminally differentiated mature B cells that secrete clonal immunoglobulins with light chain restriction. They can manifest as Solitary Plasmacytoma (SP) or Multiple Myeloma (MM).5 SP was divided into SBP and SEP. Most plasma cells are located in the bone marrow; in rare cases, they can migrate to soft tissues with the help of adhesion molecules.6 This mechanism results in an extremely low incidence of SEP. SEP is rarer than SBP, accounting for approximately 30% of all SP cases. SEP is more common in males, with a median diagnostic age of 55–60 years and an incidence rate of 0.063–0.1 per 100,000 person-years.7 Pain is the most common initial symptom of SEP and is mostly caused by compression exerted by a local mass on the affected organ.8 Swelling may appear after or coexist with pain, varying in degree depending on the size of the tumor. Pain and swelling may restrict the mobility of the surrounding joints. When a tumor compresses the surrounding blood vessels and nerves, it may cause local skin redness, numbness, sensory loss, and other symptoms. SEP in the sternoclavicular joint is extremely rare, and specialized studies or case reports on this condition are relatively scarce.
Due to the rarity of SEP, the diagnostic process for this patient was extremely challenging. The patient presented with swelling and pain in the sternoclavicular joint, redness of the skin, pain, restricted movement in the left neck and shoulder, and decreased appetite, without systemic symptoms such as fever or chest tightness. Given that the symptoms were localized to the sternoclavicular joint area and inflammatory markers—such as ESR, CRP, PCT, and interleukin-6—were significantly elevated, the initial consideration was a rheumatologic or infectious disease. After admission, comprehensive laboratory and imaging examinations were conducted, including complete blood cell analysis, inflammatory marker testing, whole-body bone ECT, PET-CT, and local biopsy. Notably, NGS testing suggested an infection with Pseudomonas aeruginosa, and cultures of the soft tissue around the sternoclavicular joint also isolated Pseudomonas aeruginosa and Proteus mirabilis. Based on these results, the initial diagnosis was sternoclavicular arthritis. Antimicrobial (antibiotic) and anti-inflammatory treatments (nonsteroidal anti-inflammatory drugs, centrally acting analgesics, and corticosteroids) were initiated. However, despite various treatment regimens, the patient’s clinical symptoms did not improve significantly and the therapeutic effect was poor. This prompted us to reevaluate the accuracy of the initial diagnosis. Considering the patient’s poor response to conventional treatment, we highly suspected that the etiology might not be a simple infectious or inflammatory lesion but rather another underlying pathological mechanism. Therefore, we focused on the local lesions and performed multiple biopsies. However, due to the insufficient number of pathological specimens obtained, a definitive diagnosis could not be made. Finally, we decided to surgically excise the lesion to obtain sufficient tissue samples for a detailed pathological examination. The excised mass was pathologically analyzed and ultimately diagnosed as SEP.
In clinical practice, sternoclavicular joint SEP are highly prone to misdiagnosis and missed diagnosis because of their rarity and nonspecific clinical manifestations. It often needs to be differentiated from rheumatological diseases, infections, and tumors. The patient in this case presented with swelling and pain in the sternoclavicular joint as the initial symptoms and was first seen in the rheumatology department. Spondyloarthritis (SpA) and SAPHO syndrome were initially considered. SpA includes ankylosing spondylitis (AS), psoriatic arthritis (PsA), reactive arthritis (ReA), and inflammatory bowel disease-associated spondyloarthritis (IBD-SpA). AS is a chronic inflammatory disease that primarily affects the spine and pelvis.9 ReA is sterile arthritis that occurs after a specific infection. PsA is a chronic inflammatory joint disease associated with psoriasis. In addition to joint manifestations, IBD-SpA also presents with clinical symptoms of inflammatory bowel disease, such as abdominal pain, diarrhea, bloody stools, and mucous stools. All the above SpA conditions responded well to anti-inflammatory and analgesic drugs. SAPHO is a rare autoinflammatory disease characterized by skin and osteoarticular manifestations.10 A retrospective study proposed that anterior chest pain was present in more than 70% of patients with SAPHO.11 The revised SAPHO diagnostic criteria in 2003 state that isolated or multifocal sterile osteitis/hyperostosis in adults, including the anterior chest wall, meets this criterion and that SAPHO can be diagnosed by excluding malignant bone and joint tumors and infections.12 Hormone therapy can significantly improve the symptoms of SAPHO. However, in this case, the patient did not respond to hormone therapy, indicating a questionable diagnosis. In terms of infection, although laboratory tests suggested inflammation, the inflammatory markers did not improve with broad-spectrum antibiotics (linezolid tablets and meropenem injections), and the pain in the sternoclavicular joint remained unrelieved, suggesting a low likelihood of infection. Despite multidisciplinary collaboration involving hematology and thoracic surgery, malignancy still needs to be highly vigilant, including primary bone tumors, bone metastasis of tumors, and lymphoma.Since multiple local biopsies and immunohistochemistry failed to provide a definitive diagnosis, the final diagnosis of SEP was confirmed by thoracoscopic lesion excision and biopsy. This case highlights the complexity of diagnosing SEP, emphasizing the need to be highly vigilant for the possibility of rare tumors when conventional treatments fail, and the importance of clarifying the diagnosis through collaboration among multiple clinical disciplines and repeat biopsy.
In cases with atypical clinical manifestations, the diagnosis of solitary extramedullary plasmacytoma (SEP) requires a comprehensive assessment that integrates results from multiple disciplines, including laboratory tests, imaging, and pathology. Laboratory tests offer advantages such as being noninvasive, rapid, widely applicable, and allowing for dynamic monitoring; however, they have limited specificity and cannot localize the lesion. Only a minority of patients with SEP have detectable monoclonal immunoglobulins in their blood or urine; in this case, neither was found in the patient’s serum or urine. Imaging techniques, including ultrasound, CT, MRI, bone ECT, and PET-CT, can identify the location and extent of the lesion but lack specificity for a definitive diagnosis. Among these, PET/CT, with its high sensitivity and specificity, can suggest the possibility of malignancy through increased metabolic activity and is used to assess treatment efficacy and identify minimal residual disease, making it valuable for the diagnosis and treatment of SEP. After imaging identifies the lesion site, a tissue sample is obtained via fine-needle aspiration or surgical biopsy for pathological examination. The definitive diagnosis of SEP relies on histopathological biopsy, wherein both histological and immunohistochemical findings must demonstrate a homogeneous infiltration of monoclonal plasma cells within a solitary lesion. These cells typically exhibit positive expression for CD138 and/or CD38.13
The prognosis of SEP is closely linked to early diagnosis and timely intervention. Large primary tumor size and lymph node metastasis have been established as adverse prognostic factors.14 As SEP is a radiosensitive tumor, radiotherapy is often recommended as the primary treatment modality.15 However, comprehensive treatment strategies should be tailored according to individual tumor characteristics, including surgical resection, radiotherapy, and systemic chemotherapy. In this case, the presence of mild bone marrow plasmacytosis (4%) and lack of CD56 expression may increase the risk of progression to multiple myeloma (MM). The optimal management strategy for SEP remains an area of ongoing research.16 For high-risk SEP patients without lymph node involvement, we recommend an individualized treatment approach. A sequential regimen combining radiotherapy and surgery may achieve better local control.17
Most progressions from SEP to MM occur within the first five years after diagnosis. We acknowledge that this study has limitations inherent to a single-case report with short-term follow-up, and the current conclusions regarding treatment efficacy and prognosis are preliminary. Therefore, the patient requires long-term close monitoring, including serial serum and urine electrophoresis with immunofixation, as well as serum free light chain analysis. Additionally, detection of high-risk cytogenetic abnormalities via interphase fluorescence in situ hybridization (FISH) in clonal plasma cells may facilitate early identification of MM18 and should be incorporated into follow-up assessments.Given the rarity of SEP, there is an urgent need for large-scale, well-designed prospective studies to further clarify optimal diagnostic and therapeutic strategies.
Swelling and pain in sternoclavicular joints are commonly associated with rheumatic, infectious, and neoplastic disorders. SEP is extremely rare and poses significant challenges for its early diagnosis. A definitive diagnosis requires the integration of histopathological features, systemic lesion evaluation, and assessment of bone marrow involvement. Timely pathological confirmation is crucial, and an excisional biopsy may be necessary to obtain adequate tissue for a definitive diagnosis. The current follow-up period for this case remains relatively short, and we will continue close monitoring to track the disease course for any progression or changes.
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Written informed consent was obtained from the patient for the publication of all the images and data included in this article. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical review and approval were not required to publish the case details in accordance with the institutional requirements.
The patient provided informed consent to publish their case details and any accompanying images.
All of us thank the patient for consent to the publication of the case.
No funding was received for this study.
The authors report no conflicts of interest in this work.
1. Basavaiah SH, Lobo FD, Philipose CS, et al. Clinicopathological spectrum of solitary plasmacytoma: a single center experience from coastal India. BMC Cancer. 2019;19(1):801. doi:10.1186/s12885-019-5976-7
2. Güler N. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 2000;88(1):240–242. doi:10.1002/(sici)1097-0142(20000101)88:1<240::aid-cncr33>3.0.co;2-w
3. Nolan KD, Mone MC, Nelson EW. Plasma cell neoplasms. Review of disease progression and report of a new variant. Surg Oncol. 2005;14(2):85–90. doi:10.1016/j.suronc.2005.05.001
4. de la Peña-Celaya JA, Aguilar-Luevano J, Alcivar-Cedeño LM, et al. Mexican concensus of multiple myeloma. Gac Med Mex. 2020;156(Suppl 1):S1–s45. Consenso Mexicano de Mieloma Múltiple. doi:10.24875/gmm.M20000393
5. Zhang X, Su L, Ran YG, et al. Extramedullary plasmacytoma of the trachea: a case report and review of the literature. Medicine. 2018;97(3):e9594. doi:10.1097/md.0000000000009594
6. Potter M. Perspectives on the origins of multiple myeloma and plasmacytomas in mice. Hematol Oncol Clin North Am. 1992;6(2):211–223.
7. Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992-2004. Br J Haematol. 2009;144(1):86–94. doi:10.1111/j.1365-2141.2008.07421.x
8. Wang J, Li J, Zhang F, Zhang P. Retroperitoneal extramedullary plasmacytoma: a case report and review of the literature. Medicine. 2018;97(46):e13281. doi:10.1097/md.0000000000013281
9. Yuan YX, Feng SR, Wu AY, et al. Influence of TNF-α inhibitors on gut microbiota and immune modulation in treating ankylosing spondylitis: insights into therapeutic mechanisms and clinical implications. J Inflamm Res. 2024;17:11741–11752. doi:10.2147/jir.S496991
10. Cheng W, Li F, Tian J, et al. New insights in the treatment of SAPHO syndrome and medication recommendations. J Inflamm Res. 2022;15:2365–2380. doi:10.2147/jir.S353539
11. Sallés M, Olivé A, Perez-Andres R, et al. The SAPHO syndrome: a clinical and imaging study. Clin Rheumatol. 2011;30(2):245–249. doi:10.1007/s10067-010-1560-x
12. Hayem G. SAPHO syndrome. Rev Prat. 2004;54(15):1635–1636. Le syndrome SAPHO.
13. Caers J, Paiva B, Zamagni E, et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: updated recommendations from a European expert panel. J Hematol Oncol. 2018;11(1):10. doi:10.1186/s13045-017-0549-1
14. Zhu Q, Zou X, You R, et al. Establishment of an innovative staging system for extramedullary plasmacytoma. BMC Cancer. 2016;16(1):777. doi:10.1186/s12885-016-2824-x
15. Mogoantă CA, Sarafoleanu C, Osman A, et al. Extramedullary plasmacytomas of the nasal cavity: case-based perspectives into optimizing the diagnostic differentiation from inflammatory polyps. Medicina. 2025;61(8). doi:10.3390/medicina61081406
16. Katano A, Sawayanagi S, Minamitani M, Ohira S, Yamashita H. Radiotherapy for solitary bony or extramedullary plasmacytoma. Cancer Diagn Progn. 2024;4(4):470–474. doi:10.21873/cdp.10350
17. Zhang T, Liu W, Liu G, Zhao T. Sequential therapy for extramedullary plasmacytoma of the palate: a rare case report with seven years of follow-up and literature review. J Cancer Res Clin Oncol. 2024;150(9):431. doi:10.1007/s00432-024-05958-1
18. Hatipoğlu U, Seyhan M, Ulas T, Dal MS, Altuntaş F. Solitary plasmacytomas: current status in 2025. Hematol Rep. 2025;17(4):32. doi:10.3390/hematolrep17040032

While market weakness had already been present coming into Friday, Trump’s post sparked a more than 12 per cent decline in bitcoin. The largest token, which had earlier this week reached an all-time high of more than US$125,000, was hovering below US$113,000 as of Friday night in New York.
Over the past 24 hours, bets worth more than US$19 billion have been wiped out, and more than 1.6 million traders liquidated, according to Coinglass data. More than US$7 billion of those positions were sold in less than one hour of trading on Friday.
“The focus now turns to counterparty exposure and whether this triggers broader market contagion,” said Brian Strugats, head trader at Multicoin Capital. He added that some estimates place total liquidations above US$30 billion.
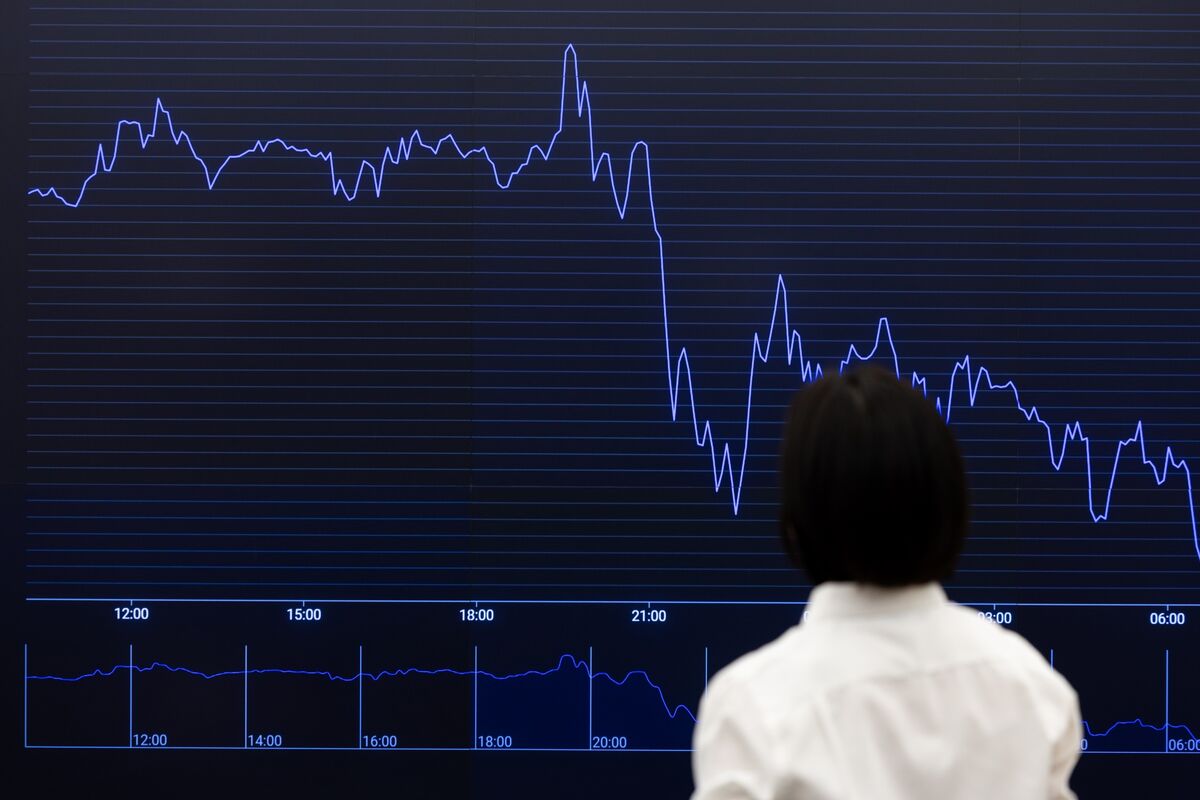
Crypto market traders were hit by record liquidations just days after Bitcoin touched an all-time high, volatility triggered in large part by the latest round of tariffs from US President Donald Trump.
Cryptocurrency prices tumbled on Friday after Trump said he would impose an additional 100% tariff on China and export controls on software. The declines precipitated, and then were made worse by what data tracker Coinglass described as “the largest liquidation event in crypto history.”

Sex And The City star Kim Cattrall says she dreams of roles that showcase women whose stories deserve to be told.
The Canadian actress, who has…

As the global population continues to age, stroke has emerged as a prevalent and significant health concern among elderly people. According to data from the Global Burden of Disease Study (GBD),1 the incidence of stroke in China is…

 Lyse Doucet
Lyse Doucet
Chief international correspondent
When President Trump announced this ceasefire deal, he hailed it, as he often does, in epic terms. This is more than…
For centuries, black seed, also known as Nigella sativa or kalonji, has been celebrated as a powerful healing spice in traditional medicine. Revered in ancient Egyptian tombs and referenced in historical medical texts, this tiny seed has been…