Using AI to transcribe speech is nothing new. Apps such as Otter.ai have been proven to be a true game changer in this regard, allowing audio containing speech to be turned into accurate, readable text in next to no time.
In many cases, however,…

Using AI to transcribe speech is nothing new. Apps such as Otter.ai have been proven to be a true game changer in this regard, allowing audio containing speech to be turned into accurate, readable text in next to no time.
In many cases, however,…

Acute pancreatitis (AP) is characterized by the dysfunction of pancreatic cellular pathways and organelles due to various etiologies, with gallstones and alcohol abuse being the most prevalent.1 This condition ultimately results in the death of pancreatic acinar cells.2 In severe instances, AP may arise as a consequence of significant complications, including local and systemic inflammatory response syndrome (SIRS) and multiple organ failure (MOF).3 AP has acute onset, severe condition and many complications, and its incidence is increasing year by year.4 A study indicated that the annual global incidence of AP is 34 cases per 100,000 individuals,5 with an overall mortality rate of approximately 5%. In cases of severe acute pancreatitis (SAP), the mortality rate may approach 20%. Currently, there is no established clinical treatment for AP. The primary pathological response in AP is the premature activation of trypsinogen, resulting in damage and death of acinar cells. Recent research has identified necroptosis as a form of regulated cell death (RCD).6 It is crucial in the mechanism of acinar cell death and the premature activation of trypsinogen.7
Necroptosis is mediated by receptor interacting protein kinase 1 (RIPK1) and receptor interacting protein kinase 3 (RIPK3). The activation of the RIPK3/mixed lineage kinase like (MLKL) pathway exhibits characteristics of both necrosis and apoptosis. Specifically, necroptosis is actively regulated by various genes and occurs in an orderly manner through the activation of specific death pathways.8–10 Salidroside (Sal) has a wide range of pharmacological activities, including Anti-inflammatory, anti-aging, antioxidant, and anti-tumor, etc.11–14 Additionally, it has been shown to decrease the activity of pancreatic enzymes during the initial stages of SAP.15,16
Based on the aforementioned evidence, we hypothesized that Sal might alleviate AP by modulating necroptosis. To test this hypothesis, the present study was designed to achieve the following specific aims: First, to investigate the therapeutic effects of Sal on pancreatic injury and systemic inflammation in a rat model of sodium taurocholate-induced AP; Second, to determine whether the protective effects of Sal are associated with the inhibition of the RIPK1/RIPK3/MLKL necroptosis pathway in both in vivo and in vitro (cerulein-stimulated AR42J cells) AP models; Finally, to explore the functional interaction between Sal and Nec-1 (a specific RIPK1 inhibitor) in order to elucidate the potential mechanism of action of Sal.
The Medical Laboratory Animal Center of Lanzhou University supplied 18 male Wistar rats with weights ranging from 250 to 280 grams. The animals were maintained in an environment characterized by alternating 12-hour light and dark cycles, with unrestricted access to food and water provided. All the rat experiments were conducted in accordance with the “Regulations on the Administration of Experimental Animals” (Lanzhou University) and were approved by the Ethics Committee of Lanzhou University Second Hospital (Approval No.D2024-410). At the same time,all methods are reported in accordance with ARRIVE guidelines.
Rat pancreatic exocrine cells-AR42J (Cellverse, iCell-r002) were cultured in AR42J specialized medium containing 20% Foetal Bovine Serum (FBS) (Procell, CM-0025) at 37°C, 5% CO2.
Firstly, the AP rat model was established by retrograde injection of sodium taurocholate solution. All rats were fasted and water-deprived overnight before the operation, and then anesthetized by intraperitoneal injection of 0.3% pentobarbital sodium (0.2 mL/10 g). Next, the rats were randomly divided into three groups (n=6): (1) Sham surgery group: only underwent laparotomy and closure sham surgery; (2) AP group: Induction of pancreatic injury by retrograde injection of 3.5% sodium taurocholate (1mL/kg, injection rate 0.1 mL/min) into the pancreaticobiliary duct. Observation after approximately 10 minutes showed congestion, edema, local bleeding, and necrosis in the pancreatic tissue, confirming the establishment of the model17,18 (3) Sal group: Based on our previous experimental results, Sal (60 mg/kg) (Medchemexpress, HY-N0109) was injected intraperitoneally 2 hours after AP modeling.15 And all animals were euthanized for sample collection at 24h post-modeling (Pentobarbital sodium, 200 mg/kg), followed by blood sample collection and serum separation through centrifugation. The pancreatic tissue was divided into three parts for pathological analysis, Western blot, and TEM detection. Finally, the animal carcasses were uniformly sent to the Gansu Province Hazardous Waste Disposal Center for processing. The final analysis included only those Wistar rats that successfully underwent the AP model induction surgery and survived the intended postoperative observation period. Rats that died during anesthesia or surgery (prior to model completion) were excluded from the analysis. Furthermore, any rat that did not exhibit a significant elevation in serum amylase levels at the predetermined time point post-modeling was also excluded, as it was deemed an induction failure. No animals were excluded from the final analysis based on these criteria.
After fixation with 4% paraformaldehyde, pancreatic tissue was embedded in paraffin and sectioned (5 μm). Hematoxylin-eosin (H&E) staining was used to assess tissue pathological damage (edema, inflammatory infiltration, acinar necrosis), with a semi-quantitative scoring system on a 0–3 scale.19 The anti-p-MLKL antibody (Affinity, AF7420, diluted 1:200) was used in immunohistochemistry (IHC) to locate p-MLKL expression, followed by DAB staining and microscopic observation. Transmission electron microscopy samples were fixed with 2.5% glutaraldehyde and ultrathin sections were observed under an electron microscope to examine the ultrastructure of mitochondria.
Serum and cell culture supernatant levels of AMY were quantified using a commercial kit (Yuanye, R22037) strictly according to the manufacturer’s instructions. Briefly, the assay involves the enzymatic hydrolysis of a defined substrate, and the resulting product is measured spectrophotometrically at a wavelength of 660 nm using a microplate reader. AMY activity is expressed in U/L. All samples were measured in duplicate. The concentrations of the inflammatory cytokines IL-6, IL-1β, and TNF-α were quantified using commercially available ELISA kits (Jonlnbio, catalog numbers JL20896, JL20884, and JL13202, respectively). The sensitivities of the assays were 1.11 pg/mL, 1.51 pg/mL, and 1.75 pg/mL for IL-6, IL-1β, and TNF-α, respectively. The detection ranges for all three cytokines were 3.12–200 pg/mL. Both intra- and inter-assay coefficients of variation were less than 10% for all assays, indicating high reproducibility and precision.
In Western blot analysis, tissues or cells are lysed with RIPA lysis buffer (containing protease/phosphatase inhibitors), and protein concentration is determined using the BCA method. 30 μg of protein was loaded into each well, separated by SDS-PAGE, and transferred to a PVDF membrane. After blocking with 5% BSA, the membrane was incubated with primary antibodies (RIPK1: Proteintech, 17519-1-AP, 1:1000; RIPK3: Bioss, bs-3551R, 1:1000; MLKL: Proteintech, 66675-1-Ig, 1:10000; p-MLKL: Affinity, AF7420, 1:1000; GAPDH: Selleck, F0003, 1:10000) and secondary antibodies. Protein expression levels were quantified by measuring the integrated density of the immunoreactive bands. Blots were imaged using a ECL chemiluminescence detection and analyzed with ImageJ software. The intensity of each target band was measured. To correct for potential variations in sample loading, the intensity of each target protein was normalized to that of the internal control (eg, GAPDH or MLKL) from the same sample. Data are presented in the bar graphs as the Mean ± SD of 4 independent experiments (due to tissue allocation constraints for protein extraction, Western blot analysis was performed on a subset of n=4 randomly selected samples per group).
First, the Cell Counting Kit-8 (CCK-8) assay was used to detect cell viability to determine the optimal drug concentrations of Sal and the RIPK1 inhibitor Necrostatin-1 (Nec-1): Cells were seeded in a 96-well plate (2×104/well), allowed to adhere overnight, and then incubated with the corresponding drugs at gradient concentrations for 8 hours. CCK-8 reagent (10 μL/well) was added, and the incubation continued at 37°C for 4 hours. The optical density (OD) at 450 nm was measured using a microplate reader to calculate cell viability. Next, AR42J cells were pre-treated with Salidroside (50 μM) or Nec-1 (10 μM) for 4 hours prior to a 4-hour stimulation with cerulein (100 nM), resulting in a total intervention period of 8 hours.20 Mitochondrial membrane potential was detected using the JC-1 dye (Beyotime, C2003S) and observed under a confocal microscope by measuring the red/green fluorescence ratio (excitation wavelength 490/525 nm, emission wavelength 530/590 nm). A decrease in the ratio indicates mitochondrial membrane potential depolarization. In the immunofluorescence experiment, after fixation and permeabilization of the cells, the anti-p-MLKL antibody (1:200) was co-stained with DAPI, and the subcellular localization of p-MLKL was observed using a confocal microscope. Only AR42J cell cultures with >95% viability were used. Any culture wells showing signs of bacterial or fungal contamination at the start of the experiment were excluded from the analysis.
The following key instruments were used in this study: Tissue FAXS PLUS microscope (Tissue FAXS PLUS, AUT), Electron microscope (Hitachi HT7800, Japan), Microplate reader (BioTek Synergy H1, USA), Confocal microscope (Zeiss LSM880, GER); ECL chemiluminescence detection (Bio-Rad ChemiDoc, SG).
Data are expressed as mean ± standard deviation (Mean ± SD). Comparisons among multiple groups were performed using one-way analysis of variance (one-way ANOVA) followed by Tukey’s multiple comparison test for post-hoc analysis. All analyses were conducted using GraphPad Prism software (Version 10). A P-value of less than 0.05 (p < 0.05) was considered statistically significant. In the figures, significance levels are denoted as follows: ns (not significant, p > 0.05), *p < 0.05, **p < 0.01, and **p < 0.001.
The changes in serum AMY and inflammatory cytokine levels indicate that Sal exerts significant anti-inflammatory effects in AP. One-way ANOVA indicated a significant difference in serum AMY levels among the groups (Figure 1A, F (2, 15) = 27.13, p < 0.001). Post-hoc Tukey’s test revealed that compared to the Sham group, AMY levels were significantly elevated in the AP model group, whereas Sal treatment reduced AMY levels by 37.4% (p < 0.01). Similarly, serum levels of the pro-inflammatory cytokines TNF-α, IL-6, and IL-1β were markedly increased in the AP group (Figure 1B; one-way ANOVA, TNF-α: F (2, 15) = 97.37, p < 0.001; IL-6: F (2, 15) = 239.6, p < 0.001; IL-1β: F (2, 15) = 99.17, p < 0.001). Sal intervention significantly reduced these cytokines by 27.6%, 23.9%, and 45.3%, respectively (all p < 0.001). H&E showed extensive pancreatic edema, inflammatory cell infiltration, and fat necrosis in the AP model group (Figure 1C). In contrast, the Sal-treated group exhibited a marked reduction in pathological damage, with a 40% decrease in histopathological scores compared to the model group (Figure 1D, p < 0.001).
|
Figure 1 Sal alleviates pancreatic injury in rats with AP. (A) Serum AMY level detection (n=6). (B) Serum TNF-α, IL-6, and IL-1β levels (ELISA detection, n=6). (C) Representative images of pancreatic tissue H&E staining (scale bar: 100 μm) (black arrows indicate the areas of acinar cell necrosis; white arrows indicate the areas of fat necrosis; red arrows indicate the areas of inflammatory cell infiltration). (D) Pancreatic pathology score (0–3 points). **p < 0.01, ***p < 0.001 compared with AP group.
|
Since Sal alleviated pancreatic damage and inflammation in AP, we further investigated whether its protective effects depend on the necroptosis pathway. Western blot analysis revealed a significant upregulation of RIPK1, RIPK3, and p-MLKL protein expression in the pancreatic tissue of the AP group (Figure 2A–C). One-way ANOVA indicated statistically significant differences among the groups for all three proteins (RIPK1: F (2, 9) = 21.70, p < 0.001; RIPK3: F (2, 9) = 29.85, p < 0.001; p-MLKL: F (2, 9) = 39.33, p < 0.001). Post hoc analysis showed that Sal treatment significantly reduced their expression levels by 21.2%, 26.1%, and 18.7%, respectively (all p < 0.05). Consistent with these results, IHC staining demonstrated strong p-MLKL immunopositivity (brown) in the perimembranous region of pancreatic cells in the AP group, which was markedly attenuated in the Sal-treated group (Figure 2D and E). TEM was employed to evaluate the ultrastructural consequences of necroptotic signaling, with a focus on mitochondrial integrity. Pancreatic acinar cells from AP model rats exhibited severe organellar damage, most notably in the form of swollen mitochondria with vacuolation and disrupted cristae (Figure 2F, middle panel), which is a characteristic manifestation of ongoing necroptosis. Treatment with Sal markedly preserved mitochondrial morphology, presenting with only mild swelling and intact cristae (Figure 2F, right panel).
 |
Figure 2 Sal inhibits the activation of the necroptosis pathway in pancreatic tissue. (A) Representative Western blot images showing protein levels of RIPK1, RIPK3, MLKL, and p-MLKL in pancreatic tissues from different experimental groups. (B) Quantitative analysis of RIPK1 and RIPK3 protein expression normalized to GAPDH (n=4). (C) Quantitative analysis of p-MLKL protein expression normalized to total MLKL (n=4). (D) Representative immunohistochemical staining of p-MLKL in pancreatic tissues (scale bar: 50 μm). Brown granules indicate positive signals. The AP group shows extensive p-MLKL expression localized around pancreatic acinar cell membranes. (E) Quantitative analysis of p-MLKL immunohistochemical staining expressed as mean optical density (OD) values (n=4). (F) Transmission electron microscopy observation of mitochondrial ultrastructure (scale bar: 1 μm). The mitochondria in the AP group showed swelling and cristae rupture, while the mitochondria in the Sal group appeared nearly normal (The black arrows indicate the mitochondrial structure of each group). *p < 0.5, **p < 0.01, ***p < 0.001 compared with AP group.
|
To further validate the anti-necroptotic and anti-inflammatory effects of Sal and elucidate its underlying mechanism, we established an in vitro AP model using cerulein-stimulated AR42J cells. First, the optimal non-cytotoxic concentrations of Sal (50 μM) and Nec-1 (10 μM) were determined via CCK-8 assay (Figure 3A and B). Cerulein (100 nM) stimulation significantly increased AMY release into the supernatant by 1.4-fold (Figure 3C; one-way ANOVA, F(3, 20) = 36.74, p < 0.001), indicating successful induction of acinar cell injury. Sal treatment alone reduced AMY levels by 15.3%, while co-treatment with Sal and Nec-1 did not yield a significant additive effect compared to Sal alone. Similarly, the release of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) was markedly elevated in the AP group (Figure 3D, one-way ANOVA, TNF-α: F (3, 20) = 37.14, p < 0.001; IL-6: F (3, 20) = 48.49, p < 0.001; IL-1β: F (3, 20) = 51.72, p < 0.001), with the most potent suppression observed in the Sal + Nec-1 co-treatment group (reductions of 29.8%, 13.2%, and 14.9%, respectively). Furthermore, cerulein induction resulted in a severe loss of mitochondrial membrane potential (indicated by a 94% decrease in red/green fluorescence ratio, p < 0.001), which was significantly restored by Sal pretreatment (Figure 3E and F, one-way ANOVA, F(3, 16) = 25.08, p < 0.001). This loss was significantly attenuated by Sal pretreatment (p < 0.05). These results confirm that Sal mitigates inflammatory damage and mitochondrial dysfunction in acinar cells under AP-like conditions.
 |
Figure 3 Sal reduces inflammatory damage in the AR42J cell AP model. (A) The effect of Sal (1–200 μM) on the viability of AR42J cells (CCK-8 assay, n=4). Choose 50 μM (where cell viability significantly increased and there was no significant toxicity) for subsequent experiments. (B) The effect of Nec-1 (1–200 μM) on cell viability (n=4). Choose 10 μM (cell viability significantly increased and no significant toxicity) for subsequent experiments. (C) AMY levels in cell supernatants (n=6). (D) TNF-α, IL-6, and IL-1β levels in cell supernatants (n=6). (E) Quantitative analysis of mitochondrial membrane potential assessed by JC-1 staining (n=5). Data are presented as the red/green fluorescence ratio. (F) Representative fluorescence images of JC-1 staining in different experimental groups (scale bar: 20 μm). Red fluorescence indicates JC-1 aggregates (high membrane potential), while green fluorescence indicates JC-1 monomers (low membrane potential). A decrease in the red/green fluorescence ratio indicates a decline in membrane potential, and Sal pretreatment partially restores the ratio. *p < 0.5, **p < 0.01, ***p < 0.001 compared with AP group.
|
We further investigated whether Sal confers protection through specific inhibition of the necroptotic pathway. Western blot analysis revealed that cerulein significantly upregulated key mediators of necroptosis, increasing RIPK1, RIPK3, and p-MLKL levels by 1.34-fold, 1.77-fold, and 1.61-fold, respectively (Figure 4A–D, one-way ANOVA, RIPK1: F(3, 12) = 10.25, p = 0.001; RIPK3: F(3, 12) = 10.47, p = 0.001; p-MLKL: F(3, 12) = 7.813, p = 0.004). Sal treatment alone significantly reduced the expression of these proteins, with p-MLKL decreasing by 34.7%. The absence of an additive effect between Sal and Nec-1 suggests that both compounds likely target the same node within the pathway. Immunofluorescence staining further demonstrated robust membrane translocation of p-MLKL in AP group cells, whereas both Sal and Nec-1 treatments markedly reduced p-MLKL membrane aggregation (Figure 4E and F, other representative fields of view for each group are shown in Supplementary Figure 1). Collectively, these data strongly support our hypothesis that Sal alleviates cerulein-induced AP injury in AR42J cells by inhibiting the RIPK1/RIPK3/MLKL-mediated necroptosis pathway.
 |
Figure 4 Sal reduces necroptosis in AR42J cells by inhibiting the RIPK1/RIPK3/MLKL pathway. (A) Representative Western blot images showing protein levels of RIPK1, RIPK3, MLKL and p-MLKL in different experimental groups. (B) Quantitative analysis of RIPK1 protein expression normalized to GAPDH (n=4). (C) Quantitative analysis of RIPK3 protein expression normalized to GAPDH (n=4). (D) Quantitative analysis of p-MLKL protein expression normalized to total MLKL (n=4). (E) Quantitative analysis of p-MLKL fluorescence intensity from immunofluorescence staining (n=4). (F) Representative immunofluorescence images of p-MLKL subcellular localization (scale bar: 50 μm). Red fluorescence represents p-MLKL signal, and blue represents DAPI nuclear staining. *p < 0.5, **p < 0.01, ***p < 0.001 compared with AP group.
|
The pathological process of AP involves complex mechanisms, among which necroptosis has been widely recognized as a critical contributor to cellular damage and inflammatory responses. However, its regulatory mechanisms and potential as a therapeutic target in AP remain incompletely elucidated. This study suggests that Sal significantly alleviates pancreatic injury in both rat AP models and AR42J cells by suppressing RIPK1/RIPK3/MLKL-mediated necroptosis, providing novel experimental evidence to support the potential clinical application of Sal in the management of AP (Figure 5).
 |
Figure 5 Sal inhibits the RIPK1/RIPK3/MLKL necroptosis pathway, prevents mitochondrial damage, and reduces inflammation in AP.
|
The present study indicate that Sal treatment markedly reduced serum levels of amylase (AMY) and pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) in AP rats (Figure 1A and B), consistent with previous studies reporting the protective effects of Sal against multi-organ injury through its anti-inflammatory and antioxidant properties.16,21–24 For instance, Wang et al25 showed that Sal inhibited furan-induced barrier damage and intestinal inflammation by suppressing TLR4/MyD88/NF-κB signaling, while Wang et al26 reported Sal alleviated mitochondrial dysfunction by activating the PGC-1α/Mfn2 signaling pathway, and restrained the endoplasmic reticulum stress. However, unlike these earlier studies that focused primarily on general anti-inflammatory effects, our study provides novel mechanistic insights by specifically establishing the inhibitory effect of Sal on necroptosis. Histopathological evaluation further confirmed that Sal ameliorated pancreatic tissue edema, necrosis, and inflammatory infiltration (Figure 1C and D).
The most significant finding of this study is the identification of Sal as a regulator of necroptosis. Western blot and immunohistochemical analyses revealed that Sal significantly inhibited the expression of RIPK1, RIPK3, and p-MLKL in pancreatic tissues (Figure 2A–C). TEM further demonstrated that Sal preserved mitochondrial structural integrity (Figure 2F), suggesting that its protective effects may be closely associated with the mitigation of mitochondrial dysfunction during necroptotic stress.
The RIPK1/RIPK3/MLKL axis is a well-established pathway mediating necroptosis.6,27 Our findings not only confirm the pivotal role of this pathway in AP but also provide new evidence for its pharmacological modulation. Sal significantly inhibited the activation of this pathway in both in vivo and in vitro AP models (Figures 2A and 4A). Notably, Nec-1 is a well-characterized and highly specific allosteric inhibitor of RIPK1. It functions by stabilizing RIPK1 in an inactive conformational state, thereby preventing its kinase activity and subsequent recruitment and phosphorylation of RIPK3. This specific inhibition blocks the initiation of the necroptotic cascade upstream of MLKL activation. In our study, the use of Nec-1 served a dual purpose: firstly, as a positive control to confirm the involvement of RIPK1-dependent necroptosis in our AP model, and secondly, as a pharmacological tool for pathway interrogation. The observation that the combination of Sal and Nec-1 did not produce a significant additive effect is a critical pharmacological clue. It suggests that Sal likely intersects with the necroptosis pathway at the level of RIPK1 or its upstream regulators, rather than acting on a parallel or downstream node.
Furthermore, Sal was found to restore mitochondrial membrane potential (Figure 3F), supporting the notion that it mitigates AP progression through a dual mechanism—inhibiting necroptosis and preserving mitochondrial function. This aligns with emerging studies emphasizing the crosstalk between necroptosis and mitochondrial dysfunction,28–30 although the precise mechanisms require further investigation. While the inhibition of this pathway is a major mechanism, we cannot rule out contributions from other pathways. This is now framed as a key mechanism rather than the exclusive mechanism.
Despite these advances, several limitations should be acknowledged. First, this study did not directly determine the pharmacokinetic parameters of Sal in an ascites environment. The absorption and distribution characteristics of Sal need to be further clarified in future research. Second, a limitation of our in vitro study is the lack of a Nec-1 alone treatment group, which would have served as a crucial positive control to benchmark the efficacy of Sal against a known RIPK1 inhibitor. Future studies will include Nec-1 as a standalone treatment to fully validate the model and provide a direct comparison for the potency of novel inhibitors like Sal. Third, it is important to note that although the pharmacological data we obtained using Nec-1 strongly suggest that Sal acts on the RIPK1 pathway, the lack of in vivo inhibitor experiments or a rescue experiment (eg, through RIPK1 overexpression) remains a limitation. Future studies employing genetic approaches both in vitro and in vivo will be essential to conclusively validate RIPK1 as the direct target. To further solidify our conclusions, future work will involve administering Nec-1 in a rat AP model to directly compare its efficacy with that of Sal and to investigate potential synergistic effects, or transfecting AR42J cells with RIPK1 plasmids to examine whether forced RIPK1 expression can counteract the protective effects of Sal, which would provide definitive mechanistic validation. Additionally, while our TEM analysis provided clear evidence of mitochondrial damage and its prevention by Sal, future studies could aim to capture more panoramic views to document the full spectrum of necroptotic ultrastructural features, such as plasma membrane rupture. Most importantly, while pharmacological evidence strongly suggests that Sal targets the necroptosis pathway, direct binding assays and validation using genetic knockout animal models are needed to identify its precise molecular target.
From a clinical translation perspective, the pharmacokinetic profile, bioavailability, and long-term safety of Sal require systematic evaluation. Moreover, crosstalk between necroptosis and other cell death modalities (eg, apoptosis, pyroptosis9,31,32) may influence its therapeutic efficacy. However, potential compensatory survival mechanisms resulting from excessive inhibition of cell death should be carefully monitored.
In conclusion, our study provides evidence that Sal alleviates AP by inhibiting necroptosis, likely through targeting the RIPK1/RIPK3/MLKL pathway. Our pharmacological data are consistent with RIPK1 being a potential target, although this requires direct genetic validation in future studies.
AP, Acute pancreatitis; SAP, Severe acute pancreatitis; Sal, Salidroside; Nec-1, Necrostatin-1; AMY, Amylase; RCD, Regulated cell death; RIPK, Receptor interacting protein kinase 3; MLKL, Mixed lineage kinase like; TEM, Transmission electron microscope; H&E, Hematoxylin-eosin; IF, Immunofluorescence; IHC, Immunohistochemistry; ELISA, Enzyme-linked immunosorbent assay; IL, Interleukin; TNF, Tumor necrosis factor; CCK-8, Cell Counting Kit-8; OD, Optical density.
The data are available from the corresponding author upon reasonable request.
All the rat experiments were conducted in accordance with the “Regulations on the Administration of Experimental Animals” (Lanzhou University) and were approved by the Ethics Committee of Lanzhou University Second Hospital (Approval No. D2024-410). At the same time,all methods are reported in accordance with ARRIVE guidelines.
We acknowledge the Cuiying Biomedical Research Center of the Lanzhou University Second Hospital for providing all the research equipments for this experiment.
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
This work was supported by the National Natural Science Foundation of China (82260135) and Science and Technology Program of Gansu Province (22YF7WA087).
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
1. Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386(9988):85–96. doi:10.1016/S0140-6736(14)60649-8
2. Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: a Review. JAMA. 2021;325(4):382–390. doi:10.1001/jama.2020.20317
3. Gardner TB. Acute Pancreatitis. Ann Intern Med. 2021;174(2):Itc17–itc32. doi:10.7326/AITC202102160
4. Wang GJ, Gao CF, Wei D, Wang C, Ding SQ. Acute pancreatitis: etiology and common pathogenesis. World J Gastroenterol. 2009;15(12):1427–1430. doi:10.3748/wjg.15.1427
5. Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(3):175–184. doi:10.1038/s41575-018-0087-5
6. Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18(5):1106–1121.
7. Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(8):479–496. doi:10.1038/s41575-019-0158-2
8. Tong X, Tang R, Xiao M, et al. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022;15(1):174. doi:10.1186/s13045-022-01392-3
9. Ketelut-Carneiro N, Fitzgerald KA. Apoptosis, Pyroptosis, and Necroptosis-Oh My! The Many Ways a Cell Can Die. J Mol Biol. 2022;434(4):167378. doi:10.1016/j.jmb.2021.167378
10. Khoury MK, Gupta K, Franco SR, Liu B. Necroptosis in the Pathophysiology of Disease. Am J Pathol. 2020;190(2):272–285. doi:10.1016/j.ajpath.2019.10.012
11. Liu X, Zhou M, Dai Z, et al. Salidroside alleviates ulcerative colitis via inhibiting macrophage pyroptosis and repairing the dysbacteriosis-associated Th17/Treg imbalance. Phytother Res. 2023;37(2):367–382. doi:10.1002/ptr.7636
12. Yang S, Wang L, Zeng Y, et al. Salidroside alleviates cognitive impairment by inhibiting ferroptosis via activation of the Nrf2/GPX4 axis in SAMP8 mice. Phytomedicine. 2023;114:154762. doi:10.1016/j.phymed.2023.154762
13. Huang G, Cai Y, Ren M, et al. Salidroside sensitizes Triple-negative breast cancer to ferroptosis by SCD1-mediated lipogenesis and NCOA4-mediated ferritinophagy. J Adv Res. 2024;2024:1.
14. Lei W, Chen MH, Huang ZF, et al. Salidroside protects pulmonary artery endothelial cells against hypoxia-induced apoptosis via the AhR/NF-κB and Nrf2/HO-1 pathways. Phytomedicine. 2024;128:155376. doi:10.1016/j.phymed.2024.155376
15. Ling Z, Peiwu L. Effect of salidroside on autophagy of pancreatic cells in rats with severe acute pancreatitis [J]Chin. J Emerg Med. 2021;30(1):53–58.
16. Wang X, Qian J, Meng Y, et al. Salidroside ameliorates severe acute pancreatitis-induced cell injury and pyroptosis by inactivating Akt/NF-κB and caspase-3/GSDME pathways. Heliyon. 2023;9(2):e13225. doi:10.1016/j.heliyon.2023.e13225
17. Wang X, Zhou G, Liu C, et al. Acanthopanax versus 3-Methyladenine Ameliorates Sodium Taurocholate-Induced Severe Acute Pancreatitis by Inhibiting the Autophagic Pathway in Rats. Mediators Inflamm. 2016;2016:8369704. doi:10.1155/2016/8369704
18. Wang X, Chu L, Liu C, et al. Therapeutic effects of Saussurea involucrata injection against severe acute pancreatitis- induced brain injury in rats. Biomed Pharmacother. 2018;100:564–574. doi:10.1016/j.biopha.2018.02.044
19. Wildi S, Kleeff J, Mayerle J, et al. Suppression of transforming growth factor beta signalling aborts caerulein induced pancreatitis and eliminates restricted stimulation at high caerulein concentrations. Gut. 2007;56(5):685–692. doi:10.1136/gut.2006.105833
20. Fu X, Xiu Z, Xu Q, Yue R, Xu H. Interleukin-22 Alleviates Caerulein-Induced Acute Pancreatitis by Activating AKT/mTOR Pathway. Dig Dis Sci. 2024;69(5):1691–1700. doi:10.1007/s10620-024-08360-6
21. Wang X, Qian J, Meng Y, et al. Salidroside alleviates severe acute pancreatitis-triggered pancreatic injury and inflammation by regulating miR-217-5p/YAF2 axis. Int Immunopharmacol. 2022;111:109123. doi:10.1016/j.intimp.2022.109123
22. Qian J, Wang X, Weng W, Zhou G, Zhu S, Liu C. Salidroside alleviates taurolithocholic acid 3-sulfate-induced AR42J cell injury. Biomed Pharmacother. 2021;142:112062. doi:10.1016/j.biopha.2021.112062
23. Fan H, Su BJ, Le JW, Zhu JH. Salidroside Protects Acute Kidney Injury in Septic Rats by Inhibiting Inflammation and Apoptosis. Drug Des Devel Ther. 2022;16:899–907. doi:10.2147/DDDT.S361972
24. Wu Q, Shan X, Li X, et al. Salidroside ameliorates neuroinflammation in autistic rats by inhibiting NLRP3/Caspase-1/GSDMD signal pathway. Brain Res Bull. 2025;220:111132. doi:10.1016/j.brainresbull.2024.111132
25. Wang Z, Li L, Li W, Yan H, Yuan Y. Salidroside Alleviates Furan-Induced Impaired Gut Barrier and Inflammation via Gut Microbiota-SCFA-TLR4 Signaling. J Agric Food Chem. 2024;72(29):16484–16495. doi:10.1021/acs.jafc.4c02433
26. Wang N, Gao Z, Zhan H, Jing L, Meng F, Chen M. Salidroside alleviates doxorubicin-induced hepatotoxicity via Sestrin2/AMPK-mediated pyroptotic inhibition. Food Chem Toxicol. 2025;199:115335. doi:10.1016/j.fct.2025.115335
27. He R, Wang Z, Dong S, Chen Z, Zhou W. Understanding Necroptosis in Pancreatic Diseases. Biomolecules. 2022;12(6):828. doi:10.3390/biom12060828
28. Ju IJ, Tsai BC, Kuo WW, et al. Rhodiola and Salidroside Attenuate Oxidative Stress-Triggered H9c2 Cardiomyoblast Apoptosis Through IGF1R-Induced ERK1/2 Activation. Environ Toxicol: Int J. 2024;39(11):5150–5161. doi:10.1002/tox.24372
29. Kang JS, Cho NJ, Lee SW, et al. RIPK3 causes mitochondrial dysfunction and albuminuria in diabetic podocytopathy through PGAM5-Drp1 signaling. Metabolism. 2024;159:155982. doi:10.1016/j.metabol.2024.155982
30. Ding Z, Wang R, Li Y, Wang X. MLKL activates the cGAS-STING pathway by releasing mitochondrial DNA upon necroptosis induction. Mol Cell. 2025;85(13):2610–25.e5. doi:10.1016/j.molcel.2025.06.005
31. Zheng M, Kanneganti TD. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol Rev. 2020;297(1):26–38. doi:10.1111/imr.12909
32. Samir P, Malireddi RKS, Kanneganti TD. The PANoptosome: a Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front Cell Infect Microbiol. 2020;10:238. doi:10.3389/fcimb.2020.00238

OpenAI has introduced ChatGPT Atlas, a new web browser that integrates ChatGPT into the browsing experience. Rather than functioning as a separate assistant that users have to switch to, Atlas incorporates the model throughout the…

In 2019 Chris Dalla Riva was the first hire when music-streaming service Audiomack set up its analytics team. Six years later he’s the company’s senior product manager for data and personalisation.
He has also steadily built an…
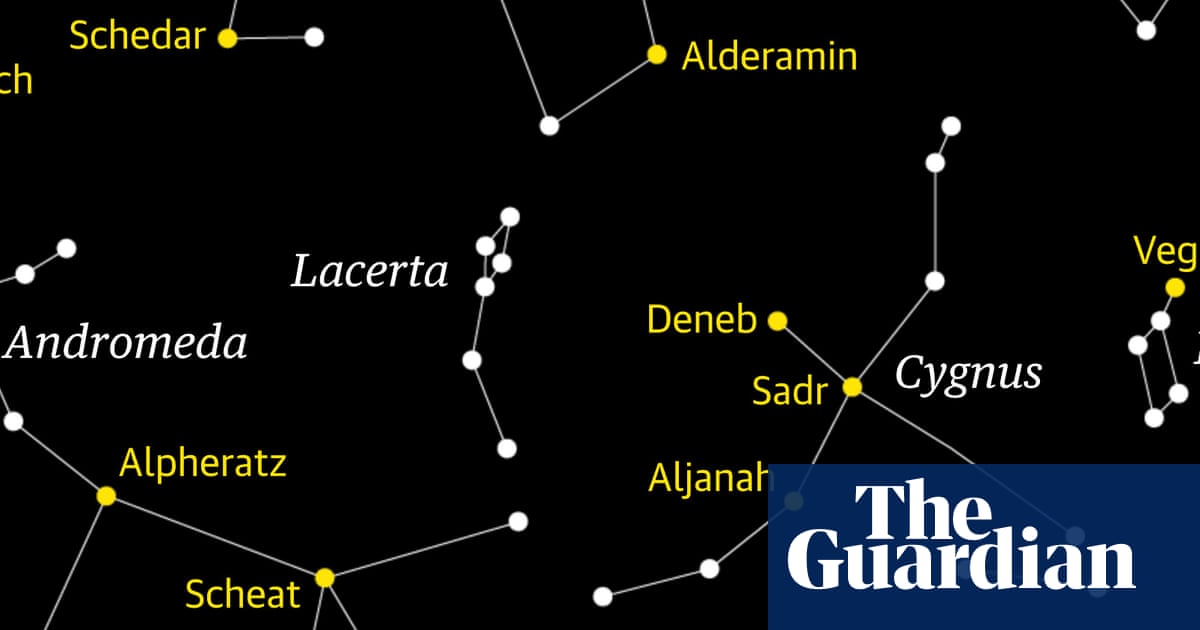
Time to track down a faint gem of the northern skies. Nestled between the bright constellations of Cygnus, the swan, and the mythical mother-daughter pair of Cassiopeia and Andromeda, Lacerta, the lizard, is admittedly a faint…

More than 100,000 cosplayers and comic book fans flocked to Excel London this weekend for MCM Comic-Con. Seeing old friends or making new ones, people young and old bond over shared interests, often feeling a sense of belonging that can be hard…

Shixuan Zhang,1 Panpan Liu,2 Sitong Liu,1 Minghuan He,1 Song Zheng,3 Xiaodong Sun,4 Ruiqun Qi,3,5 Xinghua Gao,3,5 Lili Zhu1
1Department of Dermatology, the People’s Hospital of Liaoning Province, Shenyang, Liaoning Province, 110016, People’s…



Aortic dissection (AD) is a life-threatening cardiovascular disorder with worldwide clinical significance, pathologically defined by an intimal rupture permitting blood entry into the medial layer, consequently creating separate true and false luminal spaces within the aortic wall.1 The disease progression entails substantial architectural changes in the aorta, featuring VSMC loss, ECM breakdown, and leukocyte accumulation.2 Crucially, inflammatory responses play a central mechanistic role in AD development. Invading immune cells (notably lymphocytes and macrophages) enhance protease and adhesion molecule production while releasing reactive oxygen species. These cells additionally trigger VSMC apoptosis, ultimately causing intimal deterioration – the principal pathological process driving AD formation.3 Although current management relying on surgical and endovascular techniques has advanced, the persistent lack of precise molecular targets and effective drug therapies emphasizes the critical necessity for discovering robust biomarkers to enable timely diagnosis and targeted treatment development.
Lactylation, an essential post-translational modification, significantly influences diverse biological pathways. Conventionally, lactate was viewed solely as the end-product of glycolysis.4 However, a paradigm shift occurred in 2019 when Professor Yingming Zhao’s research team identified lactylation as a novel protein modification occurring on lysine residues, thereby revolutionizing research in this field. This enzymatic process, catalyzed by specific enzymes, facilitates the attachment of lactate groups to lysine residues, subsequently modifying protein charge, structure, and function, and ultimately influencing diverse biological processes.5 The implications of lactylation extend across multiple cellular physiological activities. During tumor development, lactylation regulates essential oncogenic processes such as cancer cell metabolism, proliferation, invasion and metastatic spread via functional modulation of proteins.6 Within the cardiovascular domain, lactylation significantly contributes to various inflammatory responses and impacts cardiovascular system functionality.7–9 Moreover, this modification critically governs immune regulation, profoundly altering immune cell functionality and response dynamics.
Given lactylation’s substantial involvement in cardiovascular diseases and inflammation,10–14 coupled with the absence of reported expression and functions of lactylation-associated genes in AD, this study aims to explore potential connections between lactylation-associated genes, AD, and immunological characteristics. This investigation seeks to provide novel directions and insights for early AD diagnosis. Integrative analysis of bulk and single-cell transcriptomic data enabled identification of lactylation-associated genes in AD and uncovered their correlation with immune microenvironment characteristics.This research is anticipated to yield promising biomarkers for AD diagnosis and establish a novel approach for AD detection, thereby contributing to the theoretical foundation and technical support for early and precise diagnosis of aortic dissection.
Transcriptomic profiles from two independent GEO cohorts (GSE52093, n=12; GSE98770, n=11) were integrated for analysis. Corresponding clinical information and normalization files were also acquired from the GEO database. To ensure data consistency, batch effects were addressed through principal component analysis (PCA) and the application of the “ComBat” function from the “SVA” R package. This processing yielded a comprehensive AD cohort comprising 23 samples and 18,249 genes, suitable for subsequent analysis.
Utilizing the integrated dataset, we performed differential expression analysis employing the limma package (version 4.2.1) in RStudio. DEGs were screened using cutoff criteria of |log2FC| > 0.58 (1.5-fold) and adjusted p-value < 0.05, with analytical results presented in volcano and heatmap visualizations.15 Subsequently, systematic functional annotation of significant DEGs was performed via Gene Ontology (GO) analysis and KEGG pathway enrichment.
Based on comprehensive literature review, we identified 323 lactylation-related genes(Supplementary Data Sheet 1). Integration of differential expression results with lactylation-related genes enabled identification of lactylation-associated DEGs (LDEGs) through intersection analysis. Functional annotation of LDEGs was subsequently conducted using R-based clusterProfiler, incorporating GO enrichment and KEGG pathway analyses to delineate their biological roles. The functional enrichment results were visualized with R’s ggplot2 package to facilitate data interpretation.
To elucidate the functional characteristics and metabolic pathways of differentially expressed lactylation-associated genes, we utilized the GeneMANIA database (http://genemania.org), a comprehensive online resource for gene function prediction and interaction network construction. This platform enables rapid generation of gene interaction networks by analyzing input gene lists, thereby elucidating relationships between genes and their interaction partners. GeneMANIA integrates diverse data sources and employs sophisticated network weighting models to predict functions of unknown genes based on their interactions with functionally characterized genes. In our study, we imported the differentially expressed lactylation-related genes into this platform to construct and visualize a protein-protein interaction (PPI) network, facilitating comprehensive analysis of gene interactions and functional associations.
Three machine learning approaches were employed to analyze pre-filtered lactylation-associated DEGs for hub gene identification in AD: (1) LASSO regression via R’s “glmNETs” package; (2) RF and SVM-REF analyses using “randomForest” and “kernlab” packages respectively. Integration of feature genes from all algorithms identified lactylation-related hub genes, followed by ROC curve analysis with AUC calculation (using “pROC”) to assess diagnostic performance for AD.
Conducting a correlation analysis and visualization between the selected hub genes and other differentially expressed genes using the “corrplot” package in R. Subsequently, analyze the differential expression of each hub gene utilizing the Wilcoxon rank-sum test. Finally, perform Gene Set Enrichment Analysis (GSEA) on the Hallmark gene sets (http://software.broadinstitute.org/gsea/msigdb/) for each hub gene using the R package “clusterProfiler” to elucidate the enriched pathways and functions associated with the hub genes.
Existing evidence confirms immune cell involvement in AD pathophysiology. Using CIBERSORT, we profiled infiltration patterns of 22 immune cell subtypes in AD cohorts to examine hub gene-immune cell interactions.16 Immune cell correlations and infiltration variations were displayed via R’s “corrplot” and “ggplot2”, while Spearman analysis (“ggstatsplot”) revealed hub gene-immune cell associations, providing mechanistic insights into AD immunopathology.
External validation using the GSE153434 dataset confirmed hub gene expression profiles, with ROC analysis evaluating their diagnostic potential.
The scRNA-seq dataset (GSE213740: 6 AD cases vs 3 controls) was processed with Seurat in R. Quality control excluded cells with: >15% mitochondrial genes, >3% ribosomal genes, >0.1% erythrocyte genes, or gene counts <200/>7500. Post-QC, PCA-based dimensionality reduction preceded cell clustering (resolution=0.05) and visualization. Cluster-specific DEGs were identified using FindMarkers (log2FC>0.5, p<0.05), with top 5 markers visualized. Cellular lactylation levels were quantified via singscore for AD-normal comparisons.
The inclusion criteria for aortic dissection cases comprised patients who were: (1) diagnosed with aortic dissection through aortic CTA angiography, (2) underwent aortic artificial vessel replacement surgery, and (3) were aged over 18 years. Normal aortic tissues were collected from individuals undergoing coronary artery bypass grafting procedures. This study incorporated a total of nine clinical samples for differential gene expression validation, including six aortic dissection cases and three control cases (with aortic wall tissue obtained from the perforation site of the ascending aorta during coronary artery bypass grafting). The study protocol was conducted in strict compliance with the ethical principles outlined in the Helsinki Declaration. All experimental procedures and study protocols were reviewed and approved by the Ethics Committee of the Affiliated Hospital of Nantong University (Ethical approval number:2024-K247-01), and written informed consent was obtained from all participants prior to their inclusion in the study.
Total RNA extraction from aortic tissues was conducted with TRIzol reagent (ACCURATE BIOTECHNOLOGY). cDNA synthesis utilized HiScript II Q RT SuperMix (+gDNA wiper, Vazyme R223-01). qPCR amplification was performed with ChamQ SYBR Master Mix (Vazyme Q311-02), normalized to β-Actin expression (primers in Table 1). The 2–ΔΔCT method calculated relative expression, with p<0.05 considered statistically significant.
|
Table 1 Primer Sequences of Hub Genes
|
To advance the identification of prospective therapeutic agents for aortic dissection, this research leveraged the Drug-Gene Interaction Database (DGIdb) to pinpoint targeted drugs corresponding to critical biological targets. The drugs that received the highest scores from this prediction were subsequently subjected to validation through molecular docking experiments with those key targets. To facilitate this process, the two-dimensional structures of small molecule ligands were sourced from the PubChem database (http://pubchem.ncbi.nlm.nih.gov/). These 2D structures were then converted into three-dimensional representations using ChemOffice software, and the resulting 3D structures were saved in mol2 file format for further analysis. For the molecular docking studies, high-resolution crystal structures of the relevant protein targets were obtained from the RCSB Protein Data Bank (RCSB PDB) (http://www.rcsb.org/). These structures were utilized as the docking receptors in our simulations. To prepare the proteins for the docking process, PyMOL software was employed to eliminate water molecules and phosphate groups, resulting in cleaned protein structures that were saved as PDB files. The molecular docking itself was conducted using AutoDock Vina 1.5.6 software, which facilitated the investigation of interactions between the proteins and the ligands. Throughout this process, the structures of both the proteins and the small molecule ligands underwent several modifications; hydrogen atoms were added to the proteins, water molecules were removed, and hydrogen atoms, along with specific torsional degrees of freedom for the small molecule ligands, were carefully managed. Following these preparations, the docking box coordinates were established.Finally, by analyzing and comparing the docking scores, the most favorable conformation from the molecular simulations was ultimately identified. Visualization and analysis of the interaction patterns between the candidate compounds and critical amino acids were conducted using PyMOL and Discovery Studio 2019 software, elucidating the 2D and 3D interaction diagrams that are crucial for understanding the binding characteristics of the predicted therapeutic agents.
After integrating and removing batch effects from two AD-related datasets (GSE52093 and GSE98770), we achieved expression profiles that included 13 patients diagnosed with AD and 10 healthy individuals (Figure 1A–D). The analysis for differential expression found 423 genes that were upregulated and 470 that were downregulated in patients with AD (Figure 2A). The 50 genes with the most significant differential expression were depicted using hierarchical clustering in a heatmap (Figure 2B). Following this, GO enrichment analysis of the differentially expressed genes (Figure 3A) indicated notable enrichment in biological processes linked to “mitotic cell cycle phase transition”, cellular components related to “collagen-containing extracellular matrix”, and molecular functions primarily associated with “actin binding.” Furthermore, an analysis of pathways using the KEGG revealed significant connections between AD and both the “cell cycle” as well as the “p53 signaling pathway” (Figure 3B).
 |
Figure 1 Analysis of the merged and corrected two GEO datasets. (A and B) Expression profiles before and after raw data correction. (C and D) Differential gene expression profiles before and after data normalization.
|
 |
Figure 2 Differential expression analysis of the AD patient dataset. (A) Volcano plot of differentially expressed genes. (B) Heatmap of the top 50 differentially expressed genes.
|
 |
Figure 3 Functional enrichment analysis of differentially expressed genes. (A) GO enrichment analysis. (B) KEGG enrichment analysis.
|
Following the acquisition of expression profiles, we examined 332 lactylation-associated genes and their differential expression patterns. The analysis revealed that, in contrast to three downregulated genes, ten lactylation-related genes were significantly upregulated in Alzheimer’s disease (AD) patients (Figure 4A and B). In order to substantiate these findings further, we conducted a functional analysis on 13 genes associated with lactylation that were expressed differentially. The GO analysis revealed notable enrichment in biological processes concerning “protein localization within organelles”, molecular functions related to “binding specific to protein domains”, and cellular components tied to “histone deacetylase binding” (Figure 4C–E). Furthermore, the KEGG pathway analysis uncovered significant changes in the pathways of “vascular smooth muscle contraction” and “cellular senescence” (Figure 4F).
 |
Figure 4 Functional Analysis of Lactylation-Related Genes. (A and B) Differentially expressed genes related to lactylation. (C–E). GO enrichment analysis of lactylation-related genes. (F) KEGG enrichment analysis of lactylation-related genes.
|
To systematically identify hub genes associated with lactylation, we employed a multi-method approach. First, LASSO regression analysis was performed on the 13 differentially expressed genes, yielding 5 feature genes (Figure 5A and B). Subsequently, random forest ranking was applied to prioritize lactylation-related genes, identifying the top 10 candidates (Figure 5C). Further refinement using the SVM-REF algorithm revealed 4 feature genes (Figure 5D). Through integration of results from these three complementary methods, we identified three hub genes for lactylation: CALM1, PTBP1, and PARP1 (Figure 5E). The diagnostic potential of these hub genes was evaluated using ROC analysis, demonstrating their robust ability to distinguish AD patients from healthy controls (Figure 5F). Finally, we constructed a protein-protein interaction network for these three differentially expressed lactylation-related hub genes using the GeneMANIA database (Supplementary Figure 1).
 |
Figure 5 Identification of Lactylation Hub Genes. (A–B) LASSO regression identified 5 feature genes. (C) Random Forest ranked the importance of 10 feature genes. (D) SVM support vector machine algorithm selected 4 feature genes. (E) The intersection of feature genes obtained from the three methods yielded 3 hub genes. (F) ROC curves of the 3 genes for predicting disease occurrence.
|
After identifying the hub genes, we performed comprehensive correlation analyses between these three hub genes and all other genes, visualizing the top three genes showing significant positive and negative correlations. Our findings revealed that RILPL1, PLS3, and GABARAPL2 exhibited significant positive correlations with CALM1, while TRIP12, SMARCC1, and CYB561D2 showed significant negative correlations with CALM1 (Supplementary Figure 2). Similarly, FES, TFDP1, and ELF3 demonstrated significant positive correlations with PARP1, whereas DNAJC24, DIXDC1, and AFTPH displayed significant negative correlations with PARP1 (Supplementary Figure 3). Furthermore, CANT1, ILF3, and XPO6 were significantly positively correlated with PTBP1, while CRBN, CD2AP, and TMEM106B showed significant negative correlations with PTBP1 (Supplementary Figure 4).
Subsequently, we investigated the roles of CALM1, PARP1, and PTBP1 in AD by comparing their expression profiles between normal and AD groups. The findings indicated that CALM1 levels were significantly elevated in the normal group in comparison to the AD group. Conversely, expression levels of PARP1 and PTBP1 were significantly increased in the AD group when compared to the normal group (Figure 6A). Additionally, we performed Gene Set Enrichment Analysis (GSEA) on these central genes (Figure 6B). This analysis uncovered a positive correlation between CALM1 levels and the myogenesis pathway, alongside a negative correlation with the glycolysis and oxidative phosphorylation pathways. Meanwhile, PARP1 expression demonstrated positive links to the NF-κB signaling pathway and the inflammatory response pathway, while showing a negative relationship with the myogenesis pathway. In a similar manner, PTBP1 expression was positively associated with both the NF-κB signaling and inflammatory response pathways, but negatively associated with the myogenesis pathway.
 |
Figure 6 Characterization of Hub Gene Expression and GSEA Enrichment Analysis. (A) Expression of hub genes, with red indicating the normal group and blue representing the AD group. (B) GSEA enrichment results of hub genes.
|
We conducted a comprehensive analysis of immune cell infiltration levels in patients with aortic dissection (AD) and healthy controls. The results revealed that healthy individuals exhibited significantly higher infiltration levels of γδT cells and resting mast cells compared to the AD group, while showing significantly lower levels of resting natural killer cell infiltration (Figure 7A and C). To further investigate the interrelationships among immune cells, we performed correlation analysis to elucidate potential interactions and their implications for immune-inflammatory mechanisms in AD (Figure 7B).
 |
Figure 7 Immunoinfiltration Analysis. (A) The relative proportions of 22 immune cell subsets in all samples from the AD dataset. (B) The correlations of the 22 immune cell subsets. (C) Differences in the levels of 22 immune cell types between the normal group and the AD group.
|
Our analysis demonstrated a positive correlation between γδT cells and both M1 macrophages and resting mast cells, suggesting potential synergistic interactions and functional interdependence among these cell populations in the immune response to aortic dissection. Conversely, we observed negative correlations between resting natural killer cells and both resting mast cells and γδT cells, potentially reflecting disease-induced imbalances in the immune system’s inflammatory response, immune regulation, and immune surveillance capabilities. These findings highlight the crucial role of immune cell interactions in the pathogenesis and progression of aortic dissection.
Furthermore, we identified significant correlations between the three hub genes and specific immune cell populations (Figure 8). CALM1 expression showed a significant negative correlation with plasma cell infiltration, while demonstrating positive correlations with CD8-positive T cells, resting mast cells, and γδT cells. PARP1 expression exhibited a negative correlation with γδT cell infiltration and positive correlations with CD4-positive naive T cells and resting NK cells. PTBP1 expression was negatively correlated with γδT cell infiltration and positively correlated with resting NK cells, activated dendritic cells, monocytes, and naive B cells. These findings suggest complex interactions between lactylation-related genes and immune cell populations in the context of aortic dissection.
 |
Figure 8 Correlation between Hub Genes and Immune Cells.
|
To assess the reliability of hub genes related to lactylation that are differentially expressed in AD, we analyzed the expression profiles of three lactylation-associated genes—CALM1, PTBP1, and PARP1—using the independent dataset GSE153434. Our comparative analysis indicated that the expression of CALM1 was significantly reduced in the AD group when compared to the normal control group. In contrast, we found that PTBP1 expression was significantly increased in the AD group relative to the normal controls (Figure 9A and B). Interestingly, PARP1 expression did not demonstrate any notable differences between the two groups (Figure 9C). The receiver operating characteristic (ROC) curve analysis revealed that both CALM1 and PTBP1 had strong diagnostic capabilities, with area under the curve (AUC) values of 0.93 and 0.83, respectively. On the other hand, PARP1 exhibited minimal diagnostic value, yielding an AUC of 0.50 (Figure 9D).
 |
Figure 9 External Dataset Validation of Hub Genes. (A–C) Expression characteristics of hub genes in the external dataset, with red representing the normal group and blue representing the AD group. (D) ROC curves of hub genes for predicting disease occurrence in the external dataset.
|
The single-cell dataset GSE213740, comprising six aortic dissection samples and three normal controls, was obtained from the GEO database. Initial quality control measures were implemented, with Figure 10A and B illustrating the distribution of gene counts (nFeature), sequencing depth (nCount), and mitochondrial gene percentage (percent.mt). For subsequent analysis, the top 2000 highly variable genes were selected, with the top 10 most variable genes displayed in Figure 10C. Based on the analysis presented in Figure 11A, we determined the optimal number of principal components (PCs) to be 9. Cluster analysis results suggested an appropriate resolution of 0.1 (Figure 11B), with marker genes for each cell population visualized through heatmap analysis (Figure 12A). Utilizing the UMAP algorithm, we classified the single-cell sequencing samples into 11 distinct clusters (Figure 12B), which were subsequently identified as 11 immune cell populations (Figure 12C). These populations included endothelial cells, smooth muscle cells, mesenchymal cells, macrophages, T cells, B cells, monocytes, mast cells, plasma cells, fibroblasts, M1 macrophages, and M2 macrophages. Differential expression analysis across all 11 cell populations identified the top five highly and lowly expressed genes in each population (Figure 13A).
 |
Figure 10 Data Quality Control. (A and B) Changes in single-cell dataset quality control before and after. (C) Top 10 highly variable gene markers.
|
 |
Figure 11 Selection of Principal Component Numbers and Resolution. (A) Heatmap is used to display the expression levels of marker genes for the top 20 PCs. (B) Clustering tree is used to select the appropriate resolution.
|
 |
Figure 12 Cell Population Clustering. (A) Heatmap of marker genes for each cell population. (B) UMAP algorithm divides cells into 11 subgroups. (C) 11 cell subgroups are annotated as 11 immune cell types.
|
 |
Figure 13 Differential Expression Analysis. (A) Differentially expressed genes (top 5) in each cell cluster. (B) Expression profiles of hub genes across different cell clusters.
|
Further investigation of lactylation hub gene expression across these 11 immune cell types revealed distinct expression patterns (Figure 13B). CALM1 exhibited high expression in smooth muscle cells, B cells, and fibroblasts, while showing low expression in endothelial cells, mesenchymal cells, mast cells, and M2 macrophages. PTBP1 demonstrated elevated expression in endothelial cells, T cells, B cells, mast cells, fibroblasts, and M1 macrophages, but was minimally expressed in smooth muscle cells, mesenchymal cells, monocytes, and M2 macrophages. Similarly, PARP1 showed high expression levels in T cells, B cells, fibroblasts, and M1 macrophages, with reduced expression observed in mesenchymal cells, monocytes, and mast cells.
To further investigate the immune-related interactions between lactylation and AD, we performed single-cell scoring analysis of the lactylation-related gene set. Our analysis revealed elevated lactylation levels in fibroblasts, smooth muscle cells, monocytes, and T cells, while other immune cell types showed no significant differences in lactylation levels (Supplementary Figure 5A). Subsequent comparative analysis of lactylation gene set scores between AD and normal samples demonstrated significantly higher lactylation scores in the AD group. This finding suggests that aortic tissues from AD patients exhibit elevated lactylation levels compared to normal controls. Furthermore, we observed significant differences in lactylation levels of immune cells between the two groups (Supplementary Figure 5B).
RT-qPCR was utilized to assess the expression profiles of CALM1, PTBP1, and PARP1 in clinical samples, with comparisons made between patients with aortic dissection (AD) (n = 6) and normal controls (n = 3). The results indicated a notable decrease in CALM1 expression within the AD cohort relative to the normal controls. Conversely, the expression levels of PTBP1 and PARP1 did not exhibit any statistically significant variations between the AD and control groups (Figure 14).
 |
Figure 14 RT-qPCR detection of hub gene mRNA expression levels. ns, no significant difference, *p < 0.05.
|
Utilizing the DGIdb database, we forecasted potential drugs aimed at the key biomarker CALM1 and ultimately pinpointed the top five highest-scoring candidate drugs (Figure 15A). Among these candidates, PRENYLAMINE, which showed the highest targeting score, was chosen for molecular docking validation against CALM1. Generally, a binding energy that falls below −5.0 kcal/mol is indicative of favorable binding activity between the ligand and its target protein, where lower binding energy values signify greater binding affinity, increased stability, and more advantageous conformational interactions. Our findings revealed that PRENYLAMINE reached a binding energy of −6.6 kcal/mol when interacting with CALM1 (Figure 15B), indicating its potential as a therapeutic option for aortic dissection.
 |
Figure 15 Drug Prediction and Molecular Docking Validation.(A) Potential targeted drugs for CALM1.(B) Molecular docking results of PRENYLAMINE with CALM1.
|
The current understanding of aortic dissection (AD) pathogenesis recognizes inflammation, apoptosis, endothelial cell dysfunction, phenotypic transformation of smooth muscle cells, and extracellular matrix degradation as critical pathological components, with emerging evidence implicating epigenetic modifications in these processes.17–21 While metabolic reprogramming and its epigenetic consequences are increasingly recognized in cardiovascular pathologies, the specific role of lactate accumulation, and its associated lactylation modifications in AD development remains unexplored. Our study bridges this knowledge gap by identifying three lactylation-associated hub genes (CALM1, PARP1, and PTBP1) through integrated RNA sequencing analysis and investigated their relationship with immune cell infiltration patterns.
Firstly, through comprehensive transcriptomic analysis, we identified CALM1 as a potential lactylation-related biomarkers distinguishing AD patients from healthy individuals. As the most evolutionarily conserved calmodulin isoform,22,23 CALM1 regulates fundamental cellular process including motility, differentiation, and proliferation,24 with established roles in oncogenesis,25 neurodegeneration,26 and fibrotic disorders.27 Mechanistically, CALM1 overexpression activates PI3K-Akt pathway, subsequently suppressing hepatic gluconeogenesis – a pathway critically involved in apoptosis regulation, oxidative stress responses, and inflammatory signaling.28
Our network analysis extends these finding by revealing novel association between CALM1 expression and mTORC1 signaling pathway, glycolysis – oxidative phosphorylation coupling, and the infiltration patterns of nearly all immune cell types. These associations suggest CALM1 as a potential orchestrator of the pro-inflammatory microenvironment characteristic of AD. However, further experimental investigations are necessary to fully elucidate the specific functions and molecular mechanisms of CALM1 in the pathogenesis of AD.
PARP1, also known as Poly(ADP-ribose) polymerase 1, is an enzyme that is highly conserved and dependent on NAD⁺. It is crucial for the regulation of various intracellular physiological processes, such as the repair of DNA damage, the regulation of gene transcription, chromatin remodeling, and the modulation of apoptosis.29–31 The relationship between PARP1 and lactate/lactylation is both intricate and significant. Lactate exerts substantial influence on PARP1, not only by directly inhibiting its activity but also by indirectly modulating its function through alterations in cellular energy metabolism and redox balance.32 In chronic inflammation, excessive PARP1 activation establishes a pathogenic feedback loop by amplifying NF-κB-mediated TNFα/IL-6 production.33,34 Our findings demonstrate elevated PARP1 expression levels in aortic tissues of AD patients compared to normal controls. This correlations between PARP1 elevation and CD4T cell/mast cell infiltration align with the previous paradigm, suggesting PARP1-mediated immune dysregulation contributes to AD progression.
The RNA-binding protein PTBP1 emerged as a third lactylation-associated factor elevated in AD tissues. As one member of heterogeneous nuclear ribonucleoprotein (hnRNP) subfamily, PTBP1is encoded on human chromosome 19p13.3.35 In the metabolism of cancer cells, PTBP1 enhances the production of the M2 isoform of pyruvate kinase (PKM2) and concurrently inhibits the expression of PKM1, resulting in a metabolic transition from oxidative phosphorylation to glycolysis, which enhances glycolytic flux and lactate accumulation, significantly influencing tumor initiation and progression.36,37 Meanwhile, recent studies identifies lactylation-modified PTBP1 as a glycolysis amplifier in glioma stem cells via PFKFB4 mRNA stabilization.38
Our single-cell analysis reveals broad PTBP1 upregulation across AD aortic cell types (endothelial cells, T cells, B cells, mast cells, fibroblasts, and M1 macrophages), suggesting it may similarly drive glycolytic flux and lactate accumulation in aortic tissues. The resultant lactylation surge could destabilize immune homeostasis through post-translational modification of regulatory proteins – a hypothesis requiring experimental verification. These findings demonstrate that PTBP1 likely functions as a promoting factor in the pathogenesis and progression of aortic dissection.
Recent advancements in epigenetic research have demonstrated that lactate-induced histone lactylation plays a significant role in modulating cellular physiological functions and immune cell modifications.39 To investigate this phenomenon in AD, we conducted a comprehensive analysis of cellular lactylation levels using single-cell sequencing data, comparing AD patients with healthy individuals. The results revealed significant lactylation elevation in AD lesions, particularly within fibroblasts, smooth muscle cells, and myeloid populations. While B/T lymphocytes exhibited relatively lower lactylation levels, their functional sensitivity to lactylation-mediated epigenetic changes warrants further investigation. The spatial correlation between lactate accumulation (from enhanced glycolysis) and lactylation patterns suggests a self-reinforcing metabolic-epigenetic circuit in AD pathogenesis.
Finally, we performed experimental validation of the three hub genes using clinical samples, which demonstrated statistically significant differences in CALM1 expression between AD and control groups, whereas PARP1 and PTBP1 showed no significant differential expression. This divergence highlights the complex translation from bioinformatic prediction to biomarker validation, potentially reflecting post-transcriptional regulation or tissue-specific expression patterns. These results indicate that CALM1, rather than PARP1 or PTBP1, may serve as a promising biomarker for AD diagnosis.
While our multi-modal analysis implicates lactylation in AD-associated immune dysregulation, several limitations must be acknowledged: 1) The observational nature of human tissue studies precludes causal inference; 2) Lactylation’s cell-type-specific effects require functional validation in experimental models; 3) The diagnostic utility of lactylation markers needs prospective clinical evaluation.
Through a multidisciplinary investigation combining bioinformatic analyses and experimental validation, we conducted a systematic exploration of lactylation-mediated regulatory mechanisms in AD pathogenesis. Our findings demonstrate that CALM1 emerges as a novel and promising diagnostic biomarker, showing robust discriminative capacity between AD patients and healthy controls through comprehensive validation across multiple analytical platforms. Complementing these discoveries, high-resolution single-cell RNA sequencing revealed cell type-specific lactylation patterns within the AD aortic microenvironment, delineating previously unrecognized epigenetic regulatory networks across diverse cellular subpopulations. These results substantially advance our understanding of metabolic-epigenetic crosstalk in vascular remodeling disorders, providing mechanistic insights that bridge lactylation dynamics with AD progression. The identification of CALM1 as a lactylation-associated diagnostic indicator, coupled with our comprehensive cellular atlas of lactylation modifications, establishes a critical foundation for future investigations into AD’s molecular pathology. This work not only elucidates new pathophysiological dimensions of aortic dissection but also presents tangible translational applications, potentially enabling the development of lactylation-targeted diagnostic panels and therapeutic interventions for this life-threatening cardiovascular condition.
The datasets supporting the conclusions of this article are available in the GEO database, https://www.ncbi.nlm.nih.gov/geo/.
All experimental procedures and study protocols were reviewed and approved by the Ethics Committee of the Affiliated Hospital of Nantong University.All studies were conducted in accordance.
This work was supported by grants from Jiangsu Provincial Research Hospital (YJXYY202204-YSB58).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Zhiming Yu, Yue Pan and Xiaoyu Qian contribute equally to this article as co-first author.
1. Vilacosta I, San Román JA, Di Bartolomeo R, et al. Acute aortic syndrome revisited: jacc state-of-the-art review. J Am Coll Cardiol. 2021;78(21):2106–2125. doi:10.1016/j.jacc.2021.09.022
2. Chen Y, Zhang T, Yao F, et al. Dysregulation of interaction between LOXhigh fibroblast and smooth muscle cells contributes to the pathogenesis of aortic dissection. Theranostics. 2022;12(2):910–928. doi:10.7150/thno.66059
3. Luo F, Zhou XL, Li JJ, et al. Inflammatory response is associated with aortic dissection. Ageing Res Rev. 2009;8(1):31–35. doi:10.1016/j.arr.2008.08.001
4. Chen AN, Luo Y, Yang YH, et al. Lactylation, a novel metabolic reprogramming code: current status and prospects. Front Immunol. 2021;12:688910. doi:10.3389/fimmu.2021.688910
5. Zhang D, Tang Z, Huang H, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574(7779):575–580. doi:10.1038/s41586-019-1678-1
6. Malsi K, Xie S, Cai Y, et al. The role of lactylation in tumor growth and cancer progression. Front Oncol. 2025;15. doi:10.3389/fonc.2025.1516785
7. She H, Hu Y, Zhao G, et al. Dexmedetomidine ameliorates myocardial ischemia-reperfusion injury by inhibiting MDH2 lactylation via regulating metabolic reprogramming. Adv Sci. 2024;11(48):e2409499. doi:10.1002/advs.202409499
8. Wang J, Yang P, Yu T, et al. Lactylation of PKM2 suppresses inflammatory metabolic adaptation in pro-inflammatory macrophages. Int J Biol Sci. 2022;18(16):6210–6225. doi:10.7150/ijbs.75434
9. Wu D, Spencer CB, Ortoga L, et al. Histone lactylation-regulated METTL3 promotes ferroptosis via m6A-modification on ACSL4 in sepsis-associated lung injury. Redox Biol. 2024;74:103194. doi:10.1016/j.redox.2024.103194
10. Zhang N, Zhang Y, Xu J, et al. α-myosin heavy chain lactylation maintains sarcomeric structure and function and alleviates the development of heart failure. Cell Res. 2023;33(9):679–698. doi:10.1038/s41422-023-00844-w
11. Li X, Chen M, Chen X, et al. TRAP1 drives smooth muscle cell senescence and promotes atherosclerosis via HDAC3-primed histone H4 lysine 12 lactylation. Eur Heart J. 2024;45(39):4219–4235. doi:10.1093/eurheartj/ehae379
12. Xu X, Zhang DD, Kong P, et al. Sox10 escalates vascular inflammation by mediating vascular smooth muscle cell transdifferentiation and pyroptosis in neointimal hyperplasia. Cell Rep. 2023;42(8):112869. doi:10.1016/j.celrep.2023.112869
13. An S, Yao Y, Hu H, et al. PDHA1 hyperacetylation-mediated lactate overproduction promotes sepsis-induced acute kidney injury via Fis1 lactylation. Cell Death Dis. 2023;14(7):457. doi:10.1038/s41419-023-05952-4
14. Lin J, Ren J. Lactate-induced lactylation and cardiometabolic diseases: from epigenetic regulation to therapeutics. Biochim Biophys Acta Mol Basis Dis. 2024;1870(6):167247. doi:10.1016/j.bbadis.2024.167247
15. Phipson B, Wu D, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi:10.1093/nar/gkv007
16. Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. doi:10.1038/nmeth.3337
17. Cui H, Chen Y, Li K, et al. Untargeted metabolomics identifies succinate as a biomarker and therapeutic target in aortic aneurysm and dissection. Eur Heart J. 2021;42(42):4373–4385. doi:10.1093/eurheartj/ehab605
18. Luo S, Kong C, Zhao S, et al. Endothelial HDAC1-ZEB2-NuRD complex drives aortic aneurysm and dissection through regulation of protein S-Sulfhydration. Circulation. 2023;147(18):1382–1403. doi:10.1161/CIRCULATIONAHA.122.062743
19. Yin Z, Zhang J, Zhao M, et al. EDIL3/Del-1 prevents aortic dissection through enhancing internalization and degradation of apoptotic vascular smooth muscle cells. Autophagy. 2024;20(11):2405–2425. doi:10.1080/15548627.2024.2367191
20. Chakraborty A, Li Y, Zhang C, et al. Epigenetic induction of smooth muscle cell phenotypic alterations in aortic aneurysms and dissections. Circulation. 2023;148(12):959–977. doi:10.1161/CIRCULATIONAHA.123.063332
21. Rombouts KB, van Merrienboer TAR, Ket JCF, et al. The role of vascular smooth muscle cells in the development of aortic aneurysms and dissections. Eur J Clin Invest. 2022;52(4):e13697. doi:10.1111/eci.13697
22. Ikura M, Ames JB. Genetic polymorphism and protein conformational plasticity in the calmodulin superfamily: two ways to promote multifunctionality. Proceedings National Academy Sci United States Am. 2006;103(5):1159–1164. doi:10.1073/pnas.0508640103
23. Zhang M, Abrams C, Wang L, et al. Structural basis for calmodulin as a dynamic calcium sensor. structure. Structure. 2012;20(5):911–923. doi:10.1016/j.str.2012.03.019
24. Chin D, Means AR. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 2000;10(8):322–328. doi:10.1016/S0962-8924(00)01800-6
25. Yao M, Fu L, Liu X, et al. In-Silico multi-omics analysis of the functional significance of calmodulin 1 in multiple cancers. Front Genet. 2022;12:793508. doi:10.3389/fgene.2021.793508
26. Esteras N, Alquézar C, de la Encarnación A, et al. Calmodulin levels in blood cells as a potential biomarker of Alzheimer’s disease. Alzheimers Res Ther. 2013;5(6):55. doi:10.1186/alzrt219
27. Ji D, Chen GF, Wang JC, et al. Identification of TAF1, HNF4A, and CALM2 as potential therapeutic target genes for liver fibrosis. J Cell Physiol. 2019;234(6):9045–9051. doi:10.1002/jcp.27579
28. Ko HL, Ren EC. Functional aspects of PARP1 in DNA repair and transcription. Biomolecules. 2012;2(4):524–548. doi:10.3390/biom2040524
29. Hussain M, Khadka P, Pekhale K, et al. RECQL4 requires PARP1 for recruitment to DNA damage, and PARG dePARylation facilitates its associated role in end joining. Exp Mol Med. 2025;57(1):264–280. doi:10.1038/s12276-024-01383-z
30. Swindall AF, Stanley JA, Yang ES. PARP-1: friend or Foe of DNA damage and repair in tumorigenesis? Cancers. 2013;5(3):943–958. doi:10.3390/cancers5030943
31. Conceição CJF, Moe E, Ribeiro PA, et al. PARP1: a comprehensive review of its mechanisms, therapeutic implications and emerging cancer treatments. Biochim Biophys Acta Rev Cancer. 2025;11(2):189282. doi:10.1016/j.bbcan.2025.189282
32. Wang L, Liang C, Li F, et al. PARP1 in carcinomas and PARP1 inhibitors as antineoplastic drugs. Int J Mol Sci. 2017;18(10):2111. doi:10.3390/ijms18102111
33. Demény MA, Virág L. The PARP enzyme family and the hallmarks of cancer part 2: hallmarks related to cancer host interactions. Cancers. 2021;13(9):2057. doi:10.3390/cancers13092057
34. Won SJ, Jang BG, Yoo BH, et al. Prevention of acute/severe hypoglycemia-induced neuron death by lactate administration. J Cereb Blood Flow Metab. 2012;32(6):1086–1096. doi:10.1038/jcbfm.2012.30
35. Pina JM, Hernandez LA, Keppetipola NM. Polypyrimidine tract binding proteins PTBP1 and PTBP2 interact with distinct proteins under splicing conditions. PLoS One. 2022;17(2):e0263287. doi:10.1371/journal.pone.0263287
36. Shinohara H, Kumazaki M, Minami Y, et al. Perturbation of energy metabolism by fatty-acid derivative AIC-47 and imatinib in BCR-ABL-harboring leukemic cells. Cancer Lett. 2016;371(1):1–11. doi:10.1016/j.canlet.2015.11.020
37. He X, Arslan AD, Ho TT, et al. Involvement of polypyrimidine tract-binding protein (PTBP1) in maintaining breast cancer cell growth and malignant properties. Oncogenesis. 2014;3(1):e84. doi:10.1038/oncsis.2013.47
38. Zhou Z, Yin X, Sun H, et al. PTBP1 lactylation promotes glioma stem cell maintenance through PFKFB4-Driven glycolysis. Cancer Res. 2025;85(4):739–757. doi:10.1158/0008-5472.CAN-24-1412
39. Gu J, Zhou J, Chen Q, et al. Tumor metabolite lactate promotes tumorigenesis by modulating MOESIN lactylation and enhancing TGF-β signaling in regulatory T cells. Cell Rep. Cell Reports. 2022;39(12):110986. doi:10.1016/j.celrep.2022.110986