- The best-dressed stars from the 2025 Academy Museum Gala vogue.com.au
- Kim Kardashian Breaks Down Her Academy Museum Gala Face Mask Look Variety
- Hollywood’s biggest stars were ready to pose at the fifth Academy Museum Gala in Los Angeles on…
Blog
-
The best-dressed stars from the 2025 Academy Museum Gala – vogue.com.au
-
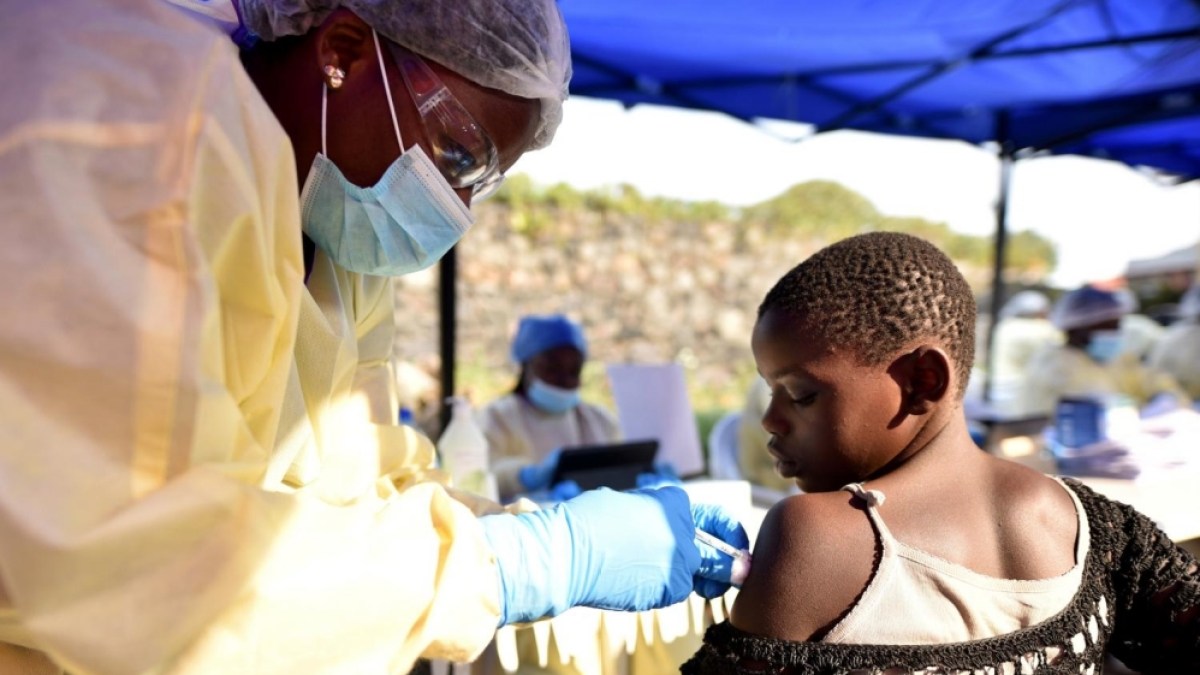
Last Ebola patient discharged in DR Congo, WHO says | Ebola News
Barring new cases, the patient’s recovery kicks off a 42-day countdown to declaring the country’s 16th outbreak over.
Published On 19 Oct 2025
The last Ebola patient in the Democratic…
Continue Reading
-

F1 LIVE: US Grand Prix 2025 reaction & result as Verstappen wins
1
Max VerstappenRed Bull
1
fastest lap 1:32.143fastest lap 1:32.143Qualifying 3, fastest lap 1:32.143
2
Lando NorrisMcLaren
4
fastest lap 1:33.224fastest lap 1:33.224fastest lap…Continue Reading
-

Bispecific ADC Iza-Bren Leads to Improved ORR Vs Chemo in Nasopharyngeal Cancer | Targeted Oncology
Patients treated with izalontamab brengitecan (iza-bren; BL-B01D1) had superior overall response rate (ORR) compared with chemotherapy in patients with recurrent or metastatic nasopharyngeal carcinoma (NPC), according to results from the BL-B01D1-301 study (NCT06118333) presented at the 2025 European Society for Medical Oncology (ESMO) Congress and published in The Lancet.1,2
The ORR by blinded independent central review was 54.6% (95% CI, 45.2%–63.8%) with iza-bren vs 27.0% (95% CI, 19.1%–36.0%) with chemotherapy, with an odds ratio of 3.3 (95% CI, 1.9–5.8; P < .0001) showing it was significantly higher in this primary end point.1
“This was the first randomized phase 3 study evaluating iza-bren in recurrent or metastatic NPC. Our study has met its primary end point for ORR, and we can see a clinically meaningful improvement in progression-free survival [PFS] and it has a management safety profile,” said Huaqiang Zhou, MD, of Sun Yat-sen University Cancer Center in Guangzhou, China, in his presentation.1
Approximately 20% to 30% of patients with NPC have recurrent or distant metastases, and current treatment options have low response rates. Iza-bren is a potentially first-in-class topoisomerase 1 inhibitor-based EGFR and HER3 bispecific antibody-drug conjugate (ADC).
The multicenter, randomized, open-label, phase 3 BL-B01D1-301 trial was designed to investigate this agent in patients who had previously received at least 2 lines of systemic chemotherapy including at least 1 platinum-containing regimen and a PD-1 or PD-L1 inhibitor. The primary end points were ORR and overall survival (OS), with secondary end points including progression-free survival, duration of response (DOR), and safety.
Patients were enrolled in 55 hospitals in China. They were stratified by number of prior lines of platinum-based treatment, ECOG performance status of 0 vs 1, and presence/absence of liver metastases.
Of 522 patients who were screened, 386 were randomly assigned on a 1:1 basis with 191 receiving 2.5 mg/kg iza-bren on days 1 and 8 of a 3-week cycle with 195 receiving physician’s choice of chemotherapy.1
The median age of patients was 50.0 in the treatment arm and 49.0 in the chemotherapy arm, with the majority being male in each arm (85.3% and 81.0%, respectively). The majority had ECOG performance status of 1 (75.9% in both arms). Over half of patients in both arms had received 2 prior lines of therapy with the rest having received at least 3 lines. The majority had received 2 prior lines of chemotherapy, with 48.2% of each arm having received 2 prior lines of platinum-based chemotherapy. Prior radiotherapy had been used in 89.5% of the experimental arm and 88.2% of the control arm.
Metastases were present at baseline in the liver, bones, and lungs in 47.6%, 49.2%, and 46.6% of the experimental arm and 48.7%, 46.7%, and 37.4% of the control arm.
Results were reported at median follow-up of 7.66 months for iza-bren and 7.10 months for chemotherapy. There was 1 complete response in the iza-bren arm and none in the control arm. The disease control rate was 82.4% with iza-bren vs 69.6% with chemotherapy. All subgroups favored iza-bren in this analysis.
The median DOR was 8.5 months for iza-bren vs 4.8 months for physician’s choice of chemotherapy (HR, 0.43; 95% CI, 0.22–0.83). The median PFS was 8.38 months with iza-bren vs 4.34 months for chemotherapy (HR, 0.44; 95% CI, 0.32–0.62), and this trend was consistent across subgroups. At this time, OS was not mature.
Treatment-related adverse events (TRAEs) of grade 3 or higher were reported in 79.9% of patients receiving iza-bren vs 61.6% of those receiving chemotherapy. Serious TRAEs occurred in 43.4% of patients in the iza-bren arm vs 27.0% in the chemotherapy arm, and 4 (2%) treatment-related deaths occurred in the iza-bren group. Dose reductions due to TRAEs were needed in 41.8% with iza-bren vs 24.3% with chemotherapy, and TRAEs leading to dose interruption occurred in 61.4% vs 18.4%, respectively. TRAEs led to treatment discontinuation in 2.6% vs 3.2%, respectively.
Hematological AEs were reported more frequently with iza-bren vs chemotherapy including anemia in 50% vs 10% and decreased platelet count in 43% vs 7%. Decreased white blood cell count occurred in 43% vs 44% and decreased neutrophil count occurred in 38% vs 41%, respectively. According to Zhou, these were well managed by standard supportive care. The majority of nonhematologic TRAEs were grade 1 or 2, and no new safety signals were identified.
Two cases of grade 2 interstitial lung disease (ILD) occurred in the experimental arm and 2 cases of grade 3 ILD occurred in the chemotherapy arm.
“Based on this trial, iza-bren represents a potential new standard of care for heavily pretreated patients with recurrent or metastatic NPC,” concluded Zhou.
REFERENCES:
1. Yang Y, Zhou H, Tang L, et al. Iza-bren (BL-B01D1), an EGFR×HER3 bispecific antibody-drug conjugate, versus physician’s choice of chemotherapy in heavily pretreated recurrent/metastatic nasopharyngeal carcinoma: a randomized, open-label, multicenter, phase III, pivotal study (BL-B01D1-303). Presented at: 2025 European Society for Medical Oncology Congress; October 17-21, 2025; Berlin, Germany. Abstract LBA35.
2. Yang Y, Zhou H, Tang L, et al. Izalontamab brengitecan, an EGFR and HER3 bispecific antibody–drug conjugate, versus chemotherapy in heavily pretreated recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 study in China. Lancet. Published online October 19, 2025. doi:10.1016/S0140-6736(25)01954-3
Continue Reading
-

Apple Liquidates 2025 MacBook Airs, Now Cheaper Than Your Average Windows Laptop
MacBooks have always carried a premium price tag that puts them out of reach for many people who’d love to experience macOS and Apple’s ecosystem. The reality is that most decent Windows laptops hover around the $800 to $1,300 range,…
Continue Reading
-

Kohler unveils a camera for your toilet
Home goods company Kohler recently unveiled a new device called the Dekoda — a $599 camera that can be attached to your toilet bowl and take pictures of what’s inside.
CNET reports that the Dekoda analyzes these images in order to provide…
Continue Reading
-
FolderFort’s 2TB Plan Is Now Cheaper Than a Year of iCloud+ – PCMag
- FolderFort’s 2TB Plan Is Now Cheaper Than a Year of iCloud+ PCMag
- Deal Alert: 2TB of FolderFort Cloud Storage Pro is now 83% off Neowin
- Give your digital world room to grow — 10TB lifetime access for a one-time $350 fee PCWorld
- It’s 10AM: Do…
Continue Reading
-

Writers and their underworlds – Matt Pearce
Seeking one thing in literary fiction, you often find another. Shortly after Charlie Kirk’s assassination a month ago, I picked up Don DeLillo’s 1997 novel “Underworld.” Not a typical response to an assassination for a journalist,…
Continue Reading
-

Portals, Dimension, and Bites: Five Fresh Ideas from Groceryshop 2025
Portals, Dimension, and Bites: Five Fresh Ideas from Groceryshop 2025 – Event Marketer
…
Continue Reading
-

Jensen Huang says Nvidia went from 95% market share in China to 0%
Nvidia CEO Jensen Huang urged nuance when it comes to regulating China’s access to U.S. technologies that are critical to developing artificial intelligence.
In an interview with Citadel Securities on Tuesday, he warned that what harms China can often harm the U.S., and sometimes even in worse ways.
“Before we leap towards policies that are hurtful to other people, take a step back and maybe reflect on what are the policies that are helpful to America,” Huang said.
His words of caution come as Nvidia processors have become hot commodities in the AI race as well as political bargaining chips in the U.S.-China trade war.
Huang said he’d like the world to run on U.S. know-how, but noted about half the world’s AI researchers are in China.
“I think it’s a mistake to not have those researchers build AI on American technology,” he added.
Trying to strike a balance between his goal of maintaining U.S. tech supremacy along with access to China will require nuance rather than an all-or-nothing approach, Huang said. But that’s not the case now, as Nvidia is “100% out of China.”
“We went from 95% market share to 0%, and so I can’t imagine any policymaker thinking that that’s a good idea, that whatever policy we implemented caused America to lose one of the largest markets in the world,” he said.
He didn’t name names, or administrations. But the Biden administration imposed rules in 2022 to restrict the export of Nvidia’s most advanced AI chips to China, leading the company to design a processor that met the new limits.
In April, Nvidia said the Trump administration blocked the sale of some of its AI chips to China without licenses and would require them for future sales. Then in August, the administration granted export licenses for certain Nvidia and AMD chips to China in exchange for 15% of the revenues.
But Chinese regulators have reportedly told domestic tech companies not to buy Nvidia chips that were designed to meet U.S. export requirements.
Meanwhile, Beijing placed strict limits on exports of rare earths, a critical input for a wide range of advanced technologies, mimicking U.S. export rules on AI chips.
That prompted President Donald Trump to fire back with an additional 100% tariff on Chinese goods. Officials from both sides are due to resume talks this week, ahead of a planned meeting with Trump and his Chinese counterpart later this month.
For now, Huang told Citadel that all of Nvidia’s financial forecasts assume China will remain out of the picture.
“If anything happens in China, which I hope it will, it’ll be a bonus,” he said. “But it’s a large market. China is the second largest computer market in the world. It is a vibrant ecosystem. I think it’s a mistake for the United States to not participate. So hopefully we’ll continue to explain and inform and hold out hope for a change in policy.”
Continue Reading
