The award-winning broadcaster Kaye Adams has spoken of her distress after she was taken off air by BBC Radio Scotland as a result of an unspecified “conduct complaint”, resulting in relentless media speculation.
In her first public comment on…

The award-winning broadcaster Kaye Adams has spoken of her distress after she was taken off air by BBC Radio Scotland as a result of an unspecified “conduct complaint”, resulting in relentless media speculation.
In her first public comment on…

Formula One driver Liam Lawson said that he narrowly avoided a fatal collision with two marshals who ran across the racetrack at the Mexico Grand Prix.
The incident occurred on the third…

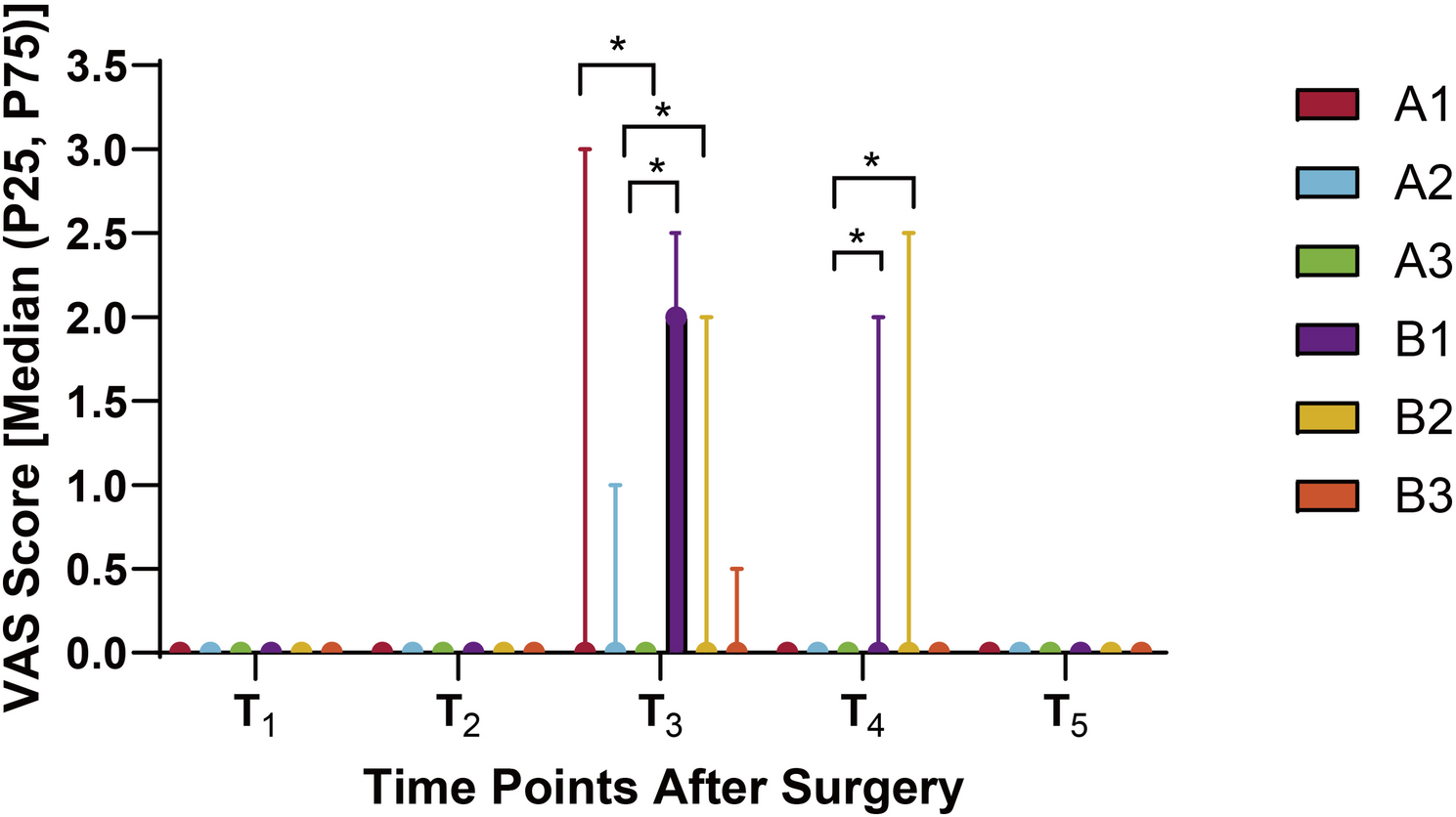
This randomized, blinded, controlled trial was conducted at First People’s Hospital of Yichang (The People’s Hospital of China Three Gorges University), approved by its Ethics Committee on December 12, 2022…
This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Ahmad F, Das D, Kommaddi RP, Diwakar L, Gowaikar R, Rupanagudi KV, Bennett DA, Ravindranath V (2018) Isoform-specific hyperactivation of calpain-2 occurs presymptomatically at the synapse in Alzheimer’s disease mice and correlates with memory deficits in human subjects. Sci Rep 8:13119. https://doi.org/10.1038/s41598-018-31073-6
Google Scholar
Aldridge JE, Horibe T, Hoogenraad NJ (2007) Discovery of genes activated by the mitochondrial unfolded protein response (mtUPR) and cognate promoter elements. PLoS ONE 2:e874. https://doi.org/10.1371/journal.pone.0000874
Google Scholar
Alldred MJ, Che S, Ginsberg SD (2008) Terminal continuation (TC) RNA amplification enables expression profiling using minute RNA input obtained from mouse brain. Int J Mol Sci 9:2091–2104
Google Scholar
Alldred MJ, Ibrahim KW, Pidikiti H, Chiosis G, Mufson EJ, Stutzmann GE, Ginsberg SD (2024) Down syndrome frontal cortex layer III and layer V pyramidal neurons exhibit lamina specific degeneration in aged individuals. Acta Neuropathol Commun 12:182. https://doi.org/10.1186/s40478-024-01891-z
Google Scholar
Alldred MJ, Penikalapati SC, Lee SH, Heguy A, Roussos P, Ginsberg SD (2021) Profiling basal forebrain cholinergic neurons reveals a molecular basis for vulnerability within the Ts65Dn model of Down syndrome and Alzheimer’s disease. Mol Neurobiol. https://doi.org/10.1007/s12035-021-02453-3
Google Scholar
Allen TG, Abogadie FC, Brown DA (2006) Simultaneous release of glutamate and acetylcholine from single magnocellular “cholinergic” basal forebrain neurons. J Neurosci 26:1588–1595. https://doi.org/10.1523/JNEUROSCI.3979-05.2006
Google Scholar
Anderson MJ (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–253. https://doi.org/10.1111/j.1541-0420.2005.00440.x
Google Scholar
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42:631–639
Google Scholar
Baumann K, Mandelkow EM, Biernat J, Piwnica-Worms H, Mandelkow E (1993) Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett 336:417–424
Google Scholar
Beck JS, Madaj Z, Cheema CT, Kara B, Bennett DA, Schneider JA, Gordon MN, Ginsberg SD, Mufson EJ, Counts SE (2022) Co-expression network analysis of frontal cortex during the progression of Alzheimer’s disease. Cereb Cortex. https://doi.org/10.1093/cercor/bhac001
Google Scholar
Beck JS, Mufson EJ, Counts SE (2015) Evidence for mitochondrial UPR gene activation in familial and sporadic Alzheimer’s disease. Curr Alzheimer Res 13:610
Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA (2018) Religious orders study and rush memory and aging project. J Alzheimers Dis 64:S161–S189. https://doi.org/10.3233/JAD-179939
Google Scholar
Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J (2002) Natural history of mild cognitive impairment in older persons. Neurology 59:198–205
Google Scholar
Berchtold NC, Coleman PD, Cribbs DH, Rogers J, Gillen DL, Cotman CW (2013) Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer’s disease. Neurobiol Aging 34:1653–1661. https://doi.org/10.1016/j.neurobiolaging.2012.11.024
Google Scholar
Bonda DJ, Castellani RJ, Zhu X, Nunomura A, Lee HG, Perry G, Smith MA (2011) A novel perspective on tau in Alzheimer’s disease. Curr Alzheimer Res 8:639–642. https://doi.org/10.2174/156720511796717131
Google Scholar
Botella-Lopez A, Burgaya F, Gavin R, Garcia-Ayllon MS, Gomez-Tortosa E, Pena-Casanova J, Urena JM, Del Rio JA, Blesa R, Soriano E et al (2006) Reelin expression and glycosylation patterns are altered in Alzheimer’s disease. Proc Natl Acad Sci U S A 103:5573–5578. https://doi.org/10.1073/pnas.0601279103
Google Scholar
Bowser R, Kordower JH, Mufson EJ (1997) A confocal microscopic analysis of galaninergic hyperinnervation of cholinergic basal forebrain neurons in Alzheimer’s disease. Brain Pathol 7:723–730
Google Scholar
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404. https://doi.org/10.1007/s00401-006-0127-z
Google Scholar
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259. https://doi.org/10.1007/bf00308809
Google Scholar
Braak H, Del Tredici K (2004) Alzheimer’s disease: intraneuronal alterations precede insoluble amyloid-beta formation. Neurobiol Aging 25:713–718. https://doi.org/10.1016/j.neurobiolaging.2003.12.015. (discussion 743-716)
Google Scholar
Carvalho C, Santos MS, Oliveira CR, Moreira PI (2015) Alzheimer’s disease and type 2 diabetes-related alterations in brain mitochondria, autophagy and synaptic markers. Biochim Biophys Acta 1852:1665–1675. https://doi.org/10.1016/j.bbadis.2015.05.001
Google Scholar
Castillo-Carranza DL, Gerson JE, Sengupta U, Guerrero-Munoz MJ, Lasagna-Reeves CA, Kayed R (2014) Specific targeting of tau oligomers in Htau mice prevents cognitive impairment and tau toxicity following injection with brain-derived tau oligomeric seeds. J Alzheimers Dis 40(Suppl 1):S97–S111. https://doi.org/10.3233/JAD-132477
Google Scholar
Chu Y, Cochran EJ, Bennett DA, Mufson EJ, Kordower JH (2001) Down-regulation of trkA mRNA within nucleus basalis neurons in individuals with mild cognitive impairment and Alzheimer’s disease. J Comp Neurol 437:296–307
Google Scholar
Clarke MTM, Brinkmalm A, Foiani MS, Woollacott IOC, Heller C, Heslegrave A, Keshavan A, Fox NC, Schott JM, Warren JD et al (2019) CSF synaptic protein concentrations are raised in those with atypical Alzheimer’s disease but not frontotemporal dementia. Alzheimers Res Ther 11:105. https://doi.org/10.1186/s13195-019-0564-2
Google Scholar
Combs B, Mueller RL, Morfini G, Brady ST, Kanaan NM (2019) Tau and axonal transport misregulation in tauopathies. Adv Exp Med Biol 1184:81–95. https://doi.org/10.1007/978-981-32-9358-8_7
Google Scholar
Counts SE, Alldred MJ, Che S, Ginsberg SD, Mufson EJ (2013) Synaptic gene dysregulation within hippocampal CA1 pyramidal neurons in mild cognitive impairment. Neuropharmacology 79C:172–179. https://doi.org/10.1016/j.neuropharm.2013.10.018
Google Scholar
Counts SE, Alldred MJ, Che S, Ginsberg SD, Mufson EJ (2014) Synaptic gene dysregulation within hippocampal CA1 pyramidal neurons in mild cognitive impairment. Neuropharmacology 79:172–179. https://doi.org/10.1016/j.neuropharm.2013.10.018
Google Scholar
Counts SE, Che S, Ginsberg SD, Mufson EJ (2011) Gender differences in neurotrophin and glutamate receptor expression in cholinergic nucleus basalis neurons during the progression of Alzheimer’s disease. J Chem Neuroanat 42:111–117. https://doi.org/10.1016/j.jchemneu.2011.02.004
Google Scholar
Counts SE, He B, Che S, Ginsberg SD, Mufson EJ (2009) Galanin fiber hyperinnervation preserves neuroprotective gene expression in cholinergic basal forebrain neurons in Alzheimer’s disease. J Alzheimers Dis 18:885–896
Google Scholar
Counts SE, He B, Che S, Ginsberg SD, Mufson EJ (2008) Galanin hyperinnervation upregulates choline acetyltransferase expression in cholinergic basal forebrain neurons in Alzheimer’s disease. Neurodegener Dis 5:228
Google Scholar
Counts SE, He B, Che S, Ikonomovic MD, DeKosky ST, Ginsberg SD, Mufson EJ (2007) Alpha7 nicotinic receptor up-regulation in cholinergic basal forebrain neurons in Alzheimer disease. Arch Neurol 64:1771–1776
Google Scholar
Counts SE, He B, Nadeem M, Wuu J, Scheff SW, Mufson EJ (2012) Hippocampal drebrin loss in mild cognitive impairment. Neurodegener Dis 10:216–219. https://doi.org/10.1159/000333122
Google Scholar
Counts SE, Mufson EJ (2005) The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J Neuropathol Exp Neurol 64:263–272
Google Scholar
Counts SE, Nadeem M, Lad SP, Wuu J, Mufson EJ (2006) Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J Neuropathol Exp Neurol 65:592–601
Google Scholar
Counts SE, Nadeem M, Wuu J, Ginsberg SD, Saragovi HU, Mufson EJ (2004) Reduction of cortical TrkA but not p75(NTR) protein in early-stage Alzheimer’s disease. Ann Neurol 56:520–531
Google Scholar
Cowan CM, Mudher A (2013) Are tau aggregates toxic or protective in tauopathies? Front Neurol 4:114. https://doi.org/10.3389/fneur.2013.00114
Google Scholar
da Rocha TJ, Silva Alves M, Guisso CC, de Andrade FM, Camozzato A, de Oliveira AA, Fiegenbaum M (2018) Association of GPX1 and GPX4 polymorphisms with episodic memory and Alzheimer’s disease. Neurosci Lett 666:32–37. https://doi.org/10.1016/j.neulet.2017.12.026
Google Scholar
Davis KL, Mohs RC, Marin D, Purohit DP, Perl DP, Lantz M, Austin G, Haroutunian V (1999) Cholinergic markers in elderly patients with early signs of Alzheimer disease. JAMA 281:1401–1406
Google Scholar
de Vries LE, Jongejan A, Monteiro Fortes J, Balesar R, Rozemuller AJM, Moerland PD, Huitinga I, Swaab DF, Verhaagen J (2024) Gene-expression profiling of individuals resilient to Alzheimer’s disease reveals higher expression of genes related to metallothionein and mitochondrial processes and no changes in the unfolded protein response. Acta Neuropathol Commun 12:68. https://doi.org/10.1186/s40478-024-01760-9
Google Scholar
DeTure MA, Dickson DW (2019) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 14:32. https://doi.org/10.1186/s13024-019-0333-5
Google Scholar
Dhar SS, Liang HL, Wong-Riley MT (2009) Nuclear respiratory factor 1 co-regulates AMPA glutamate receptor subunit 2 and cytochrome c oxidase: tight coupling of glutamatergic transmission and energy metabolism in neurons. J Neurochem 108:1595–1606
Google Scholar
Dhar SS, Wong-Riley MT (2009) Coupling of energy metabolism and synaptic transmission at the transcriptional level: role of nuclear respiratory factor 1 in regulating both cytochrome c oxidase and NMDA glutamate receptor subunit genes. J Neurosci 29:483–492
Google Scholar
Du F, Yu Q, Kanaan NM, Yan SS (2022) Mitochondrial oxidative stress contributes to the pathological aggregation and accumulation of tau oligomers in Alzheimer’s disease. Hum Mol Genet 31:2498–2507. https://doi.org/10.1093/hmg/ddab363
Google Scholar
Dujardin S, Commins C, Lathuiliere A, Beerepoot P, Fernandes AR, Kamath TV, De Los Santos MB, Klickstein N, Corjuc DL, Corjuc BT et al (2020) Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat Med 26:1256–1263. https://doi.org/10.1038/s41591-020-0938-9
Google Scholar
Falke E, Nissanov J, Mitchell TW, Bennett DA, Trojanowski JQ, Arnold SE (2003) Subicular dendritic arborization in Alzheimer’s disease correlates with neurofibrillary tangle density. Am J Pathol 163:1615–1621. https://doi.org/10.1016/S0002-9440(10)63518-3
Google Scholar
Figueiro-Silva J, Gruart A, Clayton KB, Podlesniy P, Abad MA, Gasull X, Delgado-Garcia JM, Trullas R (2015) Neuronal pentraxin 1 negatively regulates excitatory synapse density and synaptic plasticity. J Neurosci 35:5504–5521. https://doi.org/10.1523/JNEUROSCI.2548-14.2015
Google Scholar
Floyd RA (1999) Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med 222:236–245
Google Scholar
Fukutani Y, Cairns NJ, Shiozawa M, Sasaki K, Sudo S, Isaki K, Lantos PL (2000) Neuronal loss and neurofibrillary degeneration in the hippocampal cortex in late-onset sporadic Alzheimer’s disease. Psychiatry Clin Neurosci 54:523–529. https://doi.org/10.1046/j.1440-1819.2000.00747.x
Google Scholar
Furuya TK, Silva PN, Payao SL, Bertolucci PH, Rasmussen LT, De Labio RW, Braga IL, Chen ES, Turecki G, Mechawar N et al (2012) Analysis of SNAP25 mRNA expression and promoter DNA methylation in brain areas of Alzheimer’s Disease patients. Neuroscience 220:41–46. https://doi.org/10.1016/j.neuroscience.2012.06.035
Google Scholar
Gene Ontology C (2021) The gene ontology resource: enriching a GOld mine. Nucleic Acids Res 49:D325–D334. https://doi.org/10.1093/nar/gkaa1113
Google Scholar
Geula C, Nagykery N, Nicholas A, Wu CK (2008) Cholinergic neuronal and axonal abnormalities are present early in aging and in Alzheimer disease. J Neuropathol Exp Neurol 67:309–318. https://doi.org/10.1097/NEN.0b013e31816a1df3
Google Scholar
Giannakopoulos P, von Gunten A, Kovari E, Gold G, Herrmann FR, Hof PR, Bouras C (2007) Stereological analysis of neuropil threads in the hippocampal formation: relationships with Alzheimer’s disease neuronal pathology and cognition. Neuropathol Appl Neurobiol 33:334–343. https://doi.org/10.1111/j.1365-2990.2007.00827.x
Google Scholar
Ginsberg SD (2008) Transcriptional profiling of small samples in the central nervous system. Methods Mol Biol 439:147–158
Google Scholar
Ginsberg SD, Alldred MJ, Counts SE, Cataldo AM, Neve RL, Jiang Y, Wuu J, Chao MV, Mufson EJ, Nixon RA et al (2010) Microarray analysis of hippocampal CA1 neurons implicates early endosomal dysfunction during Alzheimer’s disease progression. Biol Psychiatry 68:885–893
Google Scholar
Ginsberg SD, Che S (2004) Combined histochemical staining, RNA amplification, regional, and single cell cDNA analysis within the hippocampus. Lab Invest 84:952–962
Google Scholar
Ginsberg SD, Che S, Counts SE, Mufson EJ (2006) Shift in the ratio of three-repeat tau and four-repeat tau mRNAs in individual cholinergic basal forebrain neurons in mild cognitive impairment and Alzheimer’s disease. J Neurochem 96:1401–1408
Google Scholar
Ginsberg SD, Che S, Counts SE, Mufson EJ (2006) Single cell gene expression profiling in Alzheimer’s disease. NeuroRx 3:302–318
Google Scholar
Ginsberg SD, Che S, Wuu J, Counts SE, Mufson EJ (2006) Down regulation of trk but not p75NTR gene expression in single cholinergic basal forebrain neurons mark the progression of Alzheimer’s disease. J Neurochem 97:475–487
Google Scholar
Ginsberg SD, Mufson EJ, Alldred MJ, Counts SE, Wuu J, Nixon RA, Che S (2011) Upregulation of select rab GTPases in cholinergic basal forebrain neurons in mild cognitive impairment and Alzheimer’s disease. J Chem Neuroanat 42:102–110. https://doi.org/10.1016/j.jchemneu.2011.05.012
Google Scholar
Gomez-Isla T, Frosch MP (2022) Lesions without symptoms: understanding resilience to Alzheimer disease neuropathological changes. Nat Rev Neurol 18:323–332. https://doi.org/10.1038/s41582-022-00642-9
Google Scholar
Guerrero-Munoz MJ, Gerson J, Castillo-Carranza DL (2015) Tau oligomers: the toxic player at synapses in Alzheimer’s disease. Front Cell Neurosci 9:464. https://doi.org/10.3389/fncel.2015.00464
Google Scholar
Guo Y, Chen SD, You J, Huang SY, Chen YL, Zhang Y, Wang LB, He XY, Deng YT, Zhang YR et al (2024) Multiplex cerebrospinal fluid proteomics identifies biomarkers for diagnosis and prediction of Alzheimer’s disease. Nat Hum Behav 8:2047–2066. https://doi.org/10.1038/s41562-024-01924-6
Google Scholar
Hondius DC, van Nierop P, Li KW, Hoozemans JJ, van der Schors RC, van Haastert ES, van der Vies SM, Rozemuller AJ, Smit AB (2016) Profiling the human hippocampal proteome at all pathologic stages of Alzheimer’s disease. Alzheimers Dement 12:654–668. https://doi.org/10.1016/j.jalz.2015.11.002
Google Scholar
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E et al (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8:1–13. https://doi.org/10.1016/j.jalz.2011.10.007
Google Scholar
Hyman BT, Trojanowski JQ (1997) Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 56:1095–1097
Google Scholar
Johnson ECB, Dammer EB, Duong DM, Ping L, Zhou M, Yin L, Higginbotham LA, Guajardo A, White B, Troncoso JC et al (2020) Large-scale proteomic analysis of Alzheimer’s disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat Med 26:769–780. https://doi.org/10.1038/s41591-020-0815-6
Google Scholar
Jovaisaite V, Auwerx J (2015) The mitochondrial unfolded protein response-synchronizing genomes. Curr Opin Cell Biol 33:74–81. https://doi.org/10.1016/j.ceb.2014.12.003
Google Scholar
Jovanovic JN, Sihra TS, Nairn AC, Hemmings HC Jr., Greengard P, Czernik AJ (2001) Opposing changes in phosphorylation of specific sites in synapsin I during Ca2+-dependent glutamate release in isolated nerve terminals. J Neurosci 21:7944–7953. https://doi.org/10.1523/JNEUROSCI.21-20-07944.2001
Google Scholar
Kelley CM, Ginsberg SD, Liang WS, Counts SE, Mufson EJ (2022) Posterior cingulate cortex reveals an expression profile of resilience in cognitively intact elders. Brain Commun 4:fcac162. https://doi.org/10.1093/braincomms/fcac162
Google Scholar
Kelly SC, He B, Perez SE, Ginsberg SD, Mufson EJ, Counts SE (2017) Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta Neuropathol Commun 5:8. https://doi.org/10.1186/s40478-017-0411-2
Google Scholar
Kelly SC, McKay EC, Beck JS, Collier TJ, Dorrance AM, Counts SE (2019) Locus coeruleus degeneration induces forebrain vascular pathology in a transgenic rat model of Alzheimer’s disease. J Alzheimers Dis 70:371–388. https://doi.org/10.3233/JAD-190090
Google Scholar
King D, Holt K, Toombs J, He X, Dando O, Okely JA, Tzioras M, Rose J, Gunn C, Correia A et al (2023) Synaptic resilience is associated with maintained cognition during ageing. Alzheimers Dement 19:2560–2574. https://doi.org/10.1002/alz.12894
Google Scholar
Koffie RM, Hyman BT, Spires-Jones TL (2011) Alzheimer’s disease: synapses gone cold. Mol Neurodegener 6:63. https://doi.org/10.1186/1750-1326-6-63
Google Scholar
Kurbatskaya K, Phillips EC, Croft CL, Dentoni G, Hughes MM, Wade MA, Al-Sarraj S, Troakes C, O’Neill MJ, Perez-Nievas BG et al (2016) Upregulation of calpain activity precedes tau phosphorylation and loss of synaptic proteins in Alzheimer’s disease brain. Acta Neuropathol Commun 4:34. https://doi.org/10.1186/s40478-016-0299-2
Google Scholar
Langfelder P, Horvath S (2008) WGCNA: an R package for weighted correlation network analysis. BMC Bioinf 9:559. https://doi.org/10.1186/1471-2105-9-559
Google Scholar
Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Clos AL, Jackson GR, Kayed R (2011) Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol Neurodegener 6:39. https://doi.org/10.1186/1750-1326-6-39
Google Scholar
Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Guerrero-Munoz MJ, Kiritoshi T, Neugebauer V, Jackson GR, Kayed R (2012) Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci Rep 2:700. https://doi.org/10.1038/srep00700
Google Scholar
Lautrup S, Sinclair DA, Mattson MP, Fang EF (2019) NAD(+) in brain aging and neurodegenerative disorders. Cell Metab 30:630–655. https://doi.org/10.1016/j.cmet.2019.09.001
Google Scholar
Liu Q, Wang X, Hu Y, Zhao JN, Huang CH, Li T, Zhang BG, He Y, Wu YQ, Zhang ZJ et al (2023) Acetylated tau exacerbates learning and memory impairment by disturbing with mitochondrial homeostasis. Redox Biol 62:102697. https://doi.org/10.1016/j.redox.2023.102697
Google Scholar
Marchesan E, Nardin A, Mauri S, Bernardo G, Chander V, Di Paola S, Chinellato M, von Stockum S, Chakraborty J, Herkenne S et al (2024) Activation of Ca(2+) phosphatase calcineurin regulates Parkin translocation to mitochondria and mitophagy in flies. Cell Death Differ 31:217–238. https://doi.org/10.1038/s41418-023-01251-9
Google Scholar
Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR (2006) Neuropathologic substrate of mild cognitive impairment. Arch Neurol 63:38–46
Google Scholar
Mary A, Eysert F, Checler F, Chami M (2023) Mitophagy in Alzheimer’s disease: molecular defects and therapeutic approaches. Mol Psychiatry 28:202–216. https://doi.org/10.1038/s41380-022-01631-6
Google Scholar
Mate De Gerando A, Welikovitch LA, Khasnavis A, Commins C, Glynn C, Chun JE, Perbet R, Hyman BT (2023) Tau seeding and spreading in vivo is supported by both AD-derived fibrillar and oligomeric tau. Acta Neuropathol 146:191–210. https://doi.org/10.1007/s00401-023-02600-1
Google Scholar
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269. https://doi.org/10.1016/j.jalz.2011.03.005
Google Scholar
Meftah S, Gan J (2023) Alzheimer’s disease as a synaptopathy: evidence for dysfunction of synapses during disease progression. Front Synaptic Neurosci 15:1129036. https://doi.org/10.3389/fnsyn.2023.1129036
Google Scholar
Mesulam M, Shaw P, Mash D, Weintraub S (2004) Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann Neurol 55:815–828
Google Scholar
Mesulam MM (2013) Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer’s disease. J Comp Neurol 521:4124–4144. https://doi.org/10.1002/cne.23415
Google Scholar
Mesulam MM, Mufson EJ, Levey AI, Wainer BH (1983) Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol 214:170–197
Google Scholar
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The consortium to establish a registry for Alzheimer’s disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Google Scholar
Montine TJ, Cholerton BA, Corrada MM, Edland SD, Flanagan ME, Hemmy LS, Kawas CH, White LR (2019) Concepts for brain aging: resistance, resilience, reserve, and compensation. Alzheimers Res Ther 11:22. https://doi.org/10.1186/s13195-019-0479-y
Google Scholar
Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS et al (2012) National institute on aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123:1–11. https://doi.org/10.1007/s00401-011-0910-3
Google Scholar
Mufson EJ (1997) NGF, p75NTR and trkA in Alzheimer’s disease. Promega Neural Notes, City, pp 16–19
Mufson EJ, Bothwell M, Hersh LB, Kordower JH (1989) Nerve growth factor receptor immunoreactive profiles in the normal, aged human basal forebrain: colocalization with cholinergic neurons. J Comp Neurol 285:196–217
Google Scholar
Mufson EJ, Bothwell M, Kordower JH (1989) Loss of nerve growth factor receptor-containing neurons in Alzheimer’s disease: a quantitative analysis across subregions of the basal forebrain. Exp Neurol 105:221–232
Google Scholar
Mufson EJ, Counts SE, Fahnestock M, Ginsberg SD (2007) Cholinotrophic molecular substrates of mild cognitive impairment in the elderly. Curr Alzheimer Res 4:340–350
Google Scholar
Mufson EJ, Counts SE, Ginsberg SD (2002) Gene expression profiles of cholinergic nucleus basalis neurons in Alzheimer’s disease. Neurochem Res 27:1035–1048
Google Scholar
Mufson EJ, Counts SE, Perez SE, Binder L (2005) Galanin plasticity in the cholinergic basal forebrain in Alzheimer’s disease and transgenic mice. Neuropeptides 39:232–236
Mufson EJ, Ginsberg SD, Ikonomovic MD, DeKosky ST (2003) Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J Chem Neuroanat 26:233–242
Google Scholar
Mufson EJ, Ikonomovic MD, Counts SE, Perez SE, Malek-Ahmadi M, Scheff SW, Ginsberg SD (2016) Molecular and cellular pathophysiology of preclinical Alzheimer’s disease. Behav Brain Res 311:54–69. https://doi.org/10.1016/j.bbr.2016.05.030
Google Scholar
Mufson EJ, Ma SY, Dills J, Cochran EJ, Leurgans S, Wuu J, Bennett DA, Jaffar S, Gilmor ML, Levey AI et al (2002) Loss of basal forebrain P75(NTR) immunoreactivity in subjects with mild cognitive impairment and Alzheimer’s disease. J Comp Neurol 443:136–153
Google Scholar
Mufson EJ, Ward S, Binder L (2014) Prefibrillar tau oligomers in mild cognitive impairment and Alzheimer’s disease. Neurodegener Dis 13:151–153. https://doi.org/10.1159/000353687
Google Scholar
Neff RA, Wang M, Vatansever S, Guo L, Ming C, Wang Q, Wang E, Horgusluoglu-Moloch E, Song WM, Li A et al (2021) Molecular subtyping of Alzheimer’s disease using RNA sequencing data reveals novel mechanisms and targets. Sci Adv. https://doi.org/10.1126/sciadv.abb5398
Google Scholar
Niewiadomska G, Niewiadomski W, Steczkowska M, Gasiorowska A (2021) Tau oligomers neurotoxicity. Life. https://doi.org/10.3390/life11010028
Google Scholar
Nixon RA, Saito KI, Grynspan F, Griffin WR, Katayama S, Honda T, Mohan PS, Shea TB, Beermann M (1994) Calcium-activated neutral proteinase (calpain) system in aging and Alzheimer’s disease. Ann N Y Acad Sci 747:77–91
Google Scholar
Ohrfelt A, Brinkmalm A, Dumurgier J, Brinkmalm G, Hansson O, Zetterberg H, Bouaziz-Amar E, Hugon J, Paquet C, Blennow K (2016) The pre-synaptic vesicle protein synaptotagmin is a novel biomarker for Alzheimer’s disease. Alzheimers Res Ther 8:41. https://doi.org/10.1186/s13195-016-0208-8
Google Scholar
Patel AO, Caldwell AB, Ramachandran S, Subramaniam S (2023) Endotype characterization reveals mechanistic differences across brain regions in sporadic Alzheimer’s disease. J Alzheimers Dis Rep 7:957–972. https://doi.org/10.3233/ADR-220098
Google Scholar
Pellegrino MW, Nargund AM, Haynes CM (2013) Signaling the mitochondrial unfolded protein response. Biochim Biophys Acta 1833:410–416. https://doi.org/10.1016/j.bbamcr.2012.02.019
Google Scholar
Pelucchi S, Gardoni F, Di Luca M, Marcello E (2022) Synaptic dysfunction in early phases of Alzheimer’s disease. Handb Clin Neurol 184:417–438. https://doi.org/10.1016/B978-0-12-819410-2.00022-9
Google Scholar
Perez CM, Gong Z, Yoo C, Roy D, Deoraj A, Felty Q (2024) Inhibitor of DNA binding protein 3 (ID3) and nuclear respiratory factor 1 (NRF1) mediated transcriptional gene signatures are associated with the severity of cerebral amyloid angiopathy. Mol Neurobiol 61:835–882. https://doi.org/10.1007/s12035-023-03541-2
Google Scholar
Perez-Nievas BG, Stein TD, Tai HC, Dols-Icardo O, Scotton TC, Barroeta-Espar I, Fernandez-Carballo L, de Munain EL, Perez J, Marquie M et al (2013) Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain 136:2510–2526. https://doi.org/10.1093/brain/awt171
Google Scholar
Price JL, McKeel DW Jr., Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW et al (2009) Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging 30:1026–1036
Google Scholar
Quntanilla RA, Tapia-Monsalves C (2020) The role of mitochondrial impairment in Alzheimer s disease neurodegeneration: the Tau connection. Curr Neuropharmacol 18:1076–1091. https://doi.org/10.2174/1570159X18666200525020259
Google Scholar
Reiner A, Yekutieli D, Benjamini Y (2003) Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics 19:368–375
Google Scholar
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK (2015) Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43:e47. https://doi.org/10.1093/nar/gkv007
Google Scholar
Salehi A, Verhaagen J, Dijkhuizen PA, Swaab DF (1996) Co-localization of high-affinity neurotrophin receptors in nucleus basalis of Meynert neurons and their differential reduction in Alzheimer’s disease. Neuroscience 75:373–387
Google Scholar
Samluk L, Ostapczuk P, Dziembowska M (2022) Long-term mitochondrial stress induces early steps of tau aggregation by increasing reactive oxygen species levels and affecting cellular proteostasis. Mol Biol Cell 33:ar67. https://doi.org/10.1091/mbc.E21-11-0553
Google Scholar
Sassin I, Schultz C, Thal DR, Rub U, Arai K, Braak E, Braak H (2000) Evolution of Alzheimer’s disease-related cytoskeletal changes in the basal nucleus of Meynert. Acta Neuropathol (Berl) 100:259–269
Google Scholar
Saura CA, Parra-Damas A, Enriquez-Barreto L (2015) Gene expression parallels synaptic excitability and plasticity changes in Alzheimer’s disease. Front Cell Neurosci 9:318. https://doi.org/10.3389/fncel.2015.00318
Google Scholar
Scheff SW, DeKosky ST, Price DA (1990) Quantitative assessment of cortical synaptic density in Alzheimer’s disease. Neurobiol Aging 11:29–37
Google Scholar
Scheff SW, Price DA (2003) Synaptic pathology in Alzheimer’s disease: a review of ultrastructural studies. Neurobiol Aging 24:1029–1046
Google Scholar
Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ (2007) Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 68:1501–1508
Google Scholar
Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA (2009) The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 66:200–208. https://doi.org/10.1002/ana.21706
Google Scholar
Seyfried NT, Dammer EB, Swarup V, Nandakumar D, Duong DM, Yin L, Deng Q, Nguyen T, Hales CM, Wingo T et al (2017) A multi-network approach identifies protein-specific co-expression in asymptomatic and symptomatic Alzheimer’s disease. Cell Syst 4(60–72):e64. https://doi.org/10.1016/j.cels.2016.11.006
Google Scholar
Shekari A, Fahnestock M (2019) Retrograde axonal transport of BDNF and proNGF diminishes with age in basal forebrain cholinergic neurons. Neurobiol Aging 84:131–140. https://doi.org/10.1016/j.neurobiolaging.2019.07.018
Google Scholar
Sheng B, Wang X, Su B, Lee HG, Casadesus G, Perry G, Zhu X (2012) Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J Neurochem 120:419–429. https://doi.org/10.1111/j.1471-4159.2011.07581.x
Google Scholar
Smiley JF, Mesulam MM (1999) Cholinergic neurons of the nucleus basalis of Meynert receive cholinergic, catecholaminergic and GABAergic synapses: an electron microscopic investigation in the monkey. Neuroscience 88:241–255
Google Scholar
Sorrentino V, Romani M, Mouchiroud L, Beck JS, Zhang H, D’Amico D, Moullan N, Potenza F, Schmid AW, Rietsch S et al (2017) Enhancing mitochondrial proteostasis reduces amyloid-beta proteotoxicity. Nature 552:187–193. https://doi.org/10.1038/nature25143
Google Scholar
Sultana R, Banks WA, Butterfield DA (2010) Decreased levels of PSD95 and two associated proteins and increased levels of BCl2 and caspase 3 in hippocampus from subjects with amnestic mild cognitive impairment: insights into their potential roles for loss of synapses and memory, accumulation of Abeta, and neurodegeneration in a prodromal stage of Alzheimer’s disease. J Neurosci Res 88:469–477. https://doi.org/10.1002/jnr.22227
Google Scholar
Swanson E, Breckenridge L, McMahon L, Som S, McConnell I, Bloom GS (2017) Extracellular tau oligomers induce invasion of endogenous tau into the somatodendritic compartment and axonal transport dysfunction. J Alzheimers Dis 58:803–820. https://doi.org/10.3233/JAD-170168
Google Scholar
Swerdlow RH, Burns JM, Khan SM (2010) The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimers Dis 20(Suppl 2):S265-279
Google Scholar
Sze CI, Troncoso JC, Kawas C, Mouton P, Price DL, Martin LJ (1997) Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J Neuropathol Exp Neurol 56:933–944
Google Scholar
Szklarczyk D, Kirsch R, Koutrouli M, Nastou K, Mehryary F, Hachilif R, Gable AL, Fang T, Doncheva NT, Pyysalo S et al (2023) The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res 51:D638–D646. https://doi.org/10.1093/nar/gkac1000
Google Scholar
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R (1991) Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30:572–580
Google Scholar
Tiernan CT, Combs B, Cox K, Morfini G, Brady ST, Counts SE, Kanaan NM (2016) Pseudophosphorylation of tau at S422 enhances SDS-stable dimer formation and impairs both anterograde and retrograde fast axonal transport. Exp Neurol 283:318–329. https://doi.org/10.1016/j.expneurol.2016.06.030
Google Scholar
Tiernan CT, Ginsberg SD, Guillozet-Bongaarts AL, Ward SM, He B, Kanaan NM, Mufson EJ, Binder LI, Counts SE (2016) Protein homeostasis gene dysregulation in pretangle bearing nucleus basalis neurons during the progression of Alzheimer’s disease. Neurobiol Aging 42:80–90
Google Scholar
Tiernan CT, Ginsberg SD, He B, Ward SM, Guillozet-Bongaarts AL, Kanaan NM, Mufson EJ, Counts SE (2018) Pretangle pathology within cholinergic nucleus basalis neurons coincides with neurotrophic and neurotransmitter receptor gene dysregulation during the progression of Alzheimer’s disease. Neurobiol Dis 117:125–136. https://doi.org/10.1016/j.nbd.2018.05.021
Google Scholar
Tiernan CT, Mufson EJ, Kanaan NM, Counts SE (2017) Tau oligomer pathology in nucleus basalis neurons during the progression of Alzheimer’s disease. J Neuropathol Exp Neurol 77:246
Utz J, Berner J, Munoz LE, Oberstein TJ, Kornhuber J, Herrmann M, Maler JM, Spitzer P (2021) Cerebrospinal fluid of patients with Alzheimer’s disease contains increased percentages of synaptophysin-bearing microvesicles. Front Aging Neurosci 13:682115. https://doi.org/10.3389/fnagi.2021.682115
Google Scholar
Vana L, Kanaan NM, Ugwu IC, Wuu J, Mufson EJ, Binder LI (2011) Progression of tau pathology in cholinergic basal forebrain neurons in mild cognitive impairment and Alzheimer’s disease. Am J Pathol 179:2533–2550. https://doi.org/10.1016/j.ajpath.2011.07.044
Google Scholar
Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci 29:9090–9103. https://doi.org/10.1523/JNEUROSCI.1357-09.2009
Google Scholar
Ward SM, Himmelstein DS, Lancia JK, Fu Y, Patterson KR, Binder LI (2013) TOC1: characterization of a selective oligomeric tau antibody. J Alzheimers Dis 37:593–602. https://doi.org/10.3233/JAD-131235
Google Scholar
Ward SM, Himmelstein DS, Ren Y, Fu Y, Yu XW, Roberts K, Binder LI, Sahara N (2014) TOC1: a valuable tool in assessing disease progression in the rTg4510 mouse model of tauopathy. Neurobiol Dis 67:37–48. https://doi.org/10.1016/j.nbd.2014.03.002
Google Scholar
Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR (1981) Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol 10:122–126
Google Scholar
Wiener HW, Perry RT, Chen Z, Harrell LE, Go RC (2007) A polymorphism in SOD2 is associated with development of Alzheimer’s disease. Genes Brain Behav 6:770–775. https://doi.org/10.1111/j.1601-183X.2007.00308.x
Google Scholar
Wojtas AM, Dammer EB, Guo Q, Ping L, Shantaraman A, Duong DM, Yin L, Fox EJ, Seifar F, Lee EB et al (2024) Proteomic changes in the human cerebrovasculature in Alzheimer’s disease and related tauopathies linked to peripheral biomarkers in plasma and cerebrospinal fluid. Alzheim Dement 20:4043–4065. https://doi.org/10.1002/alz.13821
Google Scholar
Wu H, Williams J, Nathans J (2014) Complete morphologies of basal forebrain cholinergic neurons in the mouse. Elife 3:e02444. https://doi.org/10.7554/eLife.02444
Google Scholar
Wu M, Zhang M, Yin X, Chen K, Hu Z, Zhou Q, Cao X, Chen Z, Liu D (2021) The role of pathological tau in synaptic dysfunction in Alzheimer’s diseases. Transl Neurodegener 10:45. https://doi.org/10.1186/s40035-021-00270-1
Google Scholar
Zheng J, Akbari M, Schirmer C, Reynaert ML, Loyens A, Lefebvre B, Buee L, Croteau DL, Galas MC, Bohr VA (2020) Hippocampal tau oligomerization early in tau pathology coincides with a transient alteration of mitochondrial homeostasis and DNA repair in a mouse model of tauopathy. Acta Neuropathol Commun 8:25. https://doi.org/10.1186/s40478-020-00896-8
Google Scholar
Zhou L, McInnes J, Wierda K, Holt M, Herrmann AG, Jackson RJ, Wang YC, Swerts J, Beyens J, Miskiewicz K et al (2017) Tau association with synaptic vesicles causes presynaptic dysfunction. Nat Commun 8:15295. https://doi.org/10.1038/ncomms15295
Google Scholar
Zhu X, Perry G, Smith MA, Wang X (2013) Abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis 33(Suppl 1):S253-262. https://doi.org/10.3233/JAD-2012-129005
Google Scholar

Victor Wembanyama continued his stunning start to the 2025 NBA season with the San Antonio Spurs on Sunday (26 October).
The French basketball star inspired the Spurs to a 118-107 win with 31 points against the Brooklyn Nets in the Western…

Shares of GameStop surged more than 7% in premarket trading early on Monday morning after the White House boosted a social media post made by the video game retailer with an AI-generated meme of President Donald Trump.
GameStop shares…