Fleetwood had started the final round in second place, two shots behind Japan’s Keita Nakajima.
New Zealander Daniel Hillier – ranked 218th in the world – leapfrogged them into a surprise lead, after moving to seven under for his round, but was…

Fleetwood had started the final round in second place, two shots behind Japan’s Keita Nakajima.
New Zealander Daniel Hillier – ranked 218th in the world – leapfrogged them into a surprise lead, after moving to seven under for his round, but was…

Q: Could you summarize the magnitude of benefit seen with Dato-DXd compared to chemotherapy in this first-line mTNBC population and comment on how clinically meaningful this is for practice?
Rebecca Dent, MD: As we know, patients with metastatic triple-negative breast cancer—especially those who are not eligible for immune checkpoint inhibition, which represents about 60% to 70% of patients we see in the first-line setting—have had chemotherapy as the standard of care for well over 10 years. In this setting, 50% of patients progress and never go on to receive second-line therapy. So there was a real need to incorporate a novel therapy into the first-line setting.
In addition, patients are receiving more treatment in the curative setting, and so if they relapse, they’ve already seen a number of different chemotherapies. What’s unique about TROPION-Breast02 is that we are incorporating an antibody-drug conjugate as the first-line therapy in patients with relapsed or de novo metastatic TNBC, or those relapsing more than 12 months after initial therapy.
Here we saw the use of Dato-DXd, a TROP2-directed antibody-drug conjugate with a potent topoisomerase payload and a stable, cleavable linker. Because of the drug-to-antibody ratio, we see potent effectiveness in terms of improvements in progression-free survival (PFS), with a safety profile that allows administration every three weeks, which is very convenient for patients.
We saw an impressive improvement in PFS—from about 5 months to over 10 months in median PFS, a delta of more than 5 months. These Kaplan-Meier curves separate very early, with a time to treatment response of 1.4 months, and the curves continue to separate and remain sustained.
We also saw an improvement in overall survival (OS). Median OS was 18.7 months with standard chemotherapy and increased to almost 2 years with Dato-DXd—a delta of 5 months, with a statistically significant hazard ratio.
The data were very consistent. In clinic, what’s extraordinary is seeing such a visible improvement in response. We saw more than a doubling of response rates with Dato-DXd compared with chemotherapy—well over 60%, a delta of more than 30%, and a tripling of the complete response rate.
What’s even more helpful in clinic is that these responses are sustained. Often, we can achieve a response, but it doesn’t last. In this trial, duration of response increased from 7 months with chemotherapy to more than 12 months with Dato-DXd—again, a 5-month improvement. So across endpoints—PFS, OS, and duration of response—we consistently saw about a 5-month delta favoring Dato-DXd.

Note: Video captions are generated with the assistance of AI and may contain errors.
John P. Berdahl, MD, a surgeon at Vance Thompson Vision and a professor at the University of South Dakota, spoke on the lens implantation strategies for patients…
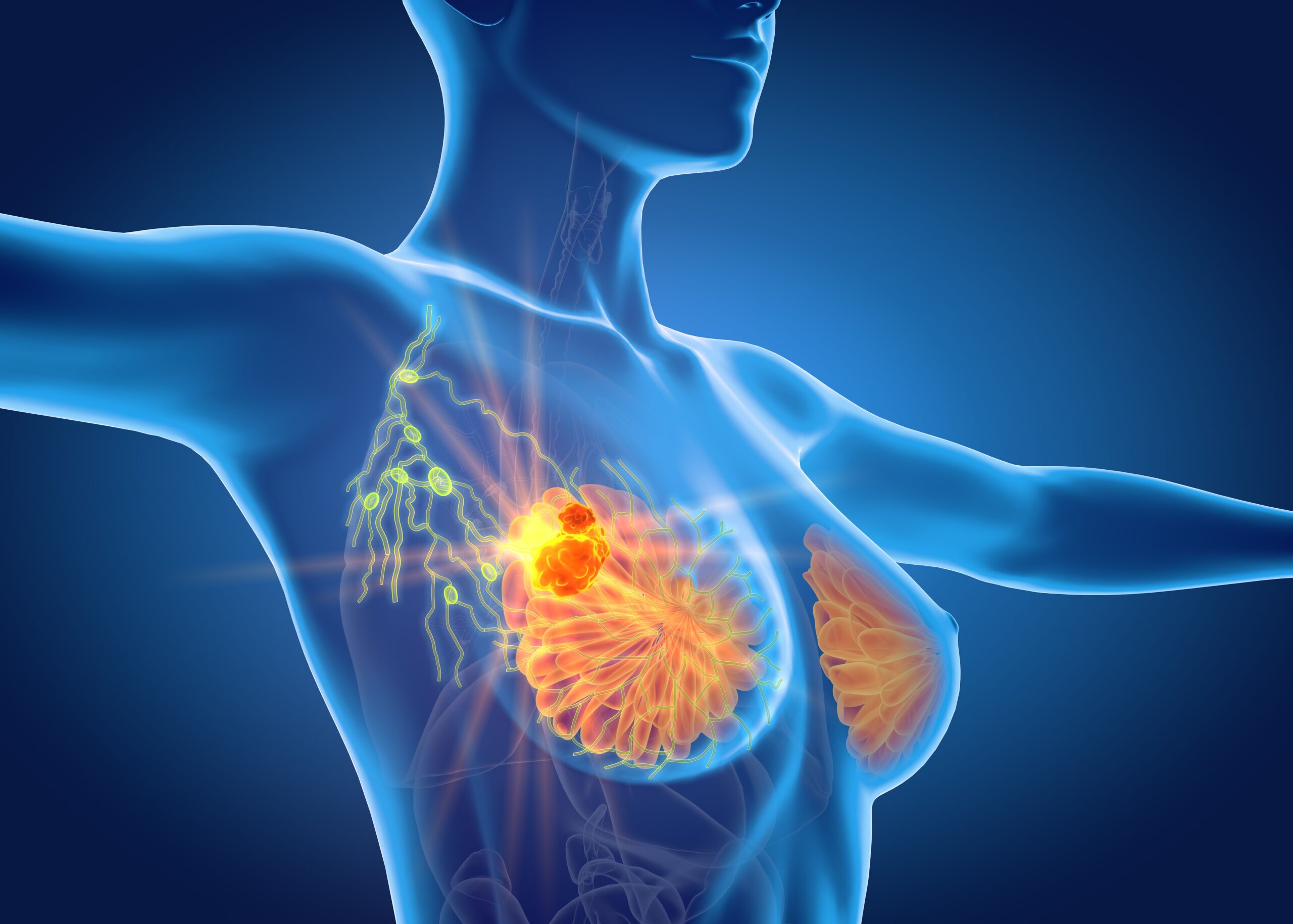
The addition of the PI3K/AKT/mTOR (PAM) inhibitor gedatolisib to fulvestrant (Faslodex), with or without palbociclib (Ibrance) led to clinically meaningful and statistically significant improvements in progression-free survival (PFS) among patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CA wild-type advanced breast cancer following progression on, or after, treatment with a CDK4/6 inhibitor and an aromatase inhibitor. 1,2
The benefits of PAM inhibition were seen in results from the phase 3 VIKTORIA-1 trial (NCT05501886) that were presented at the
“VIKTORIA-1 is the first study to demonstrate a statistically significant and clinically meaningful improvement in progression-free survival with PAM inhibition in patients with PIK3CA wild-type disease, all of whom received prior CDK4/6 inhibitor,” Sara A. Hurvitz, MD, FACP, senior vice president and director, Clinical Research Division, Fred Hutch, said during a presentation of the data.
Compared with fulvestrant alone (arm C), both the triplet of gedatolisib, fulvestrant, and palbociclib (arm A) and doublet regimen of gedatolisib plus fulvestrant (arm B) induced superior PFS. The triplet reduced the risk of disease progression by 76% compared with fulvestrant alone (median PFS 9.3 months [95% CI, 7.2-16.6] vs 2.0 months [95% CI, 1.8-2.3], respectively; hazard ratio, 0.24; 95% CI, 0.17-0.35; P <.0001). The doublet reduced the risk of progression by 67% vs fulvestrant alone (7.4 months [95% CI, 5.5-9.9] vs 2.0 months [95% CI. 1.8-2.3], respectively; hazard ratio, 0.33; 95% CI, 0.24-0.48; P <.0001).
Superior median PFS was consistent across subgroup analyses for both the triple and doublet regimens. Most noteworthy, according to Hurvitz, was that patients who received prior palbociclib benefited from the triplet regimen. “This is the first time that data in the randomized setting suggested benefit to palbociclib rechallenge,” she said.
When comparing median PFS benefit between arms A and B, the triplet regimen demonstrated higher clinical benefit in almost all subgroups, including those who were pre/perimenopausal, endocrine therapy resistant, with visceral metastases, and for those who had previously received treatment with palbociclib.
At a data cutoff of May 30, 2025, data for overall survival (OS) were immature, with only 48% of the protocol specified events, Hurvitz noted. Compared with 18.5 months (95% CI, 15.8-NE) in the monotherapy arm, the triplet and doublet regimens, respectively, demonstrated a median OS of 23.7 months (95% CI, 21.4-not estimable [NE]; hazard ratio, 0.69; 95% CI, 0.43-1.12; P = .1328) and not reached (NR; 95% CI, NR-NR; hazard ratio, 0.74; 95% CI, 0.46-1.19; P = .2122).
Sixty-three patients in the fulvestrant arm crossed over to the triplet (n = 52; 48.1%) or doublet (n = 11; 10.2%) regimens. This could impact overall survival, Hurvitz said, adding that a sensitivity analysis performed at the time of crossover showed a greater separation of the OS curves in favor of the triplet (hazard ratio, 0.60; 95% CI, 0.35-1.04; P = .0698) and doublet (hazard ratio, 0.56; 95% CI, 0.33-0.97; P = .0393) regimens.
“The study is continuing to follow patients for overall survival, with final analysis is anticipated about 48 months after the first patient was randomized, or early 2027,” she added.
Tumor response was also superior in both the triple and doublet regimens, compared with fulvestrant alone.
In the triplet arm, the ORR was 31.5%. There was 1 complete response (CR), 38 partial responses (PRs), 67 patients with stable disease (SD), and 17 patients with progressive disease (PD). Further, the clinical benefit rate (CBR; defined as CR, PR, and SD >24 weeks) among this arm was 50%, with a disease control rate (DCR; defined as CR, PR, and SD) of 85.5%. The DOR was a median of 17.5 months (95% CI, 8.8-NE).
In the doublet arm, the ORR was 28.3%, with no CRs, 32 PRs, 55 with SD, and 26 with PD. CBR and DCR were 48.7% and 77.0%, respectively, with a median DOR of 12.0 months (95% CI, 8.1-NE).
Lastly, in the monotherapy arm, the ORR was 1.0%, with no CRs, 1 PR, 40 with SD, and 62 with PD. The CBR and DCR were 11.4% and 39.0%, respectively, with the median DOR not reached.
Treatment with gedatolisib was generally well tolerated in both arms with no new safety signals, according to Hurvitz.
In total, 3 patients in the triplet arm and 4 in the doublet arm discontinued treatment due to a treatment-related adverse event (TRAE). Two deaths occurred in the triplet therapy arm.
Treatment-related AEs of interest with the triplet and doublet regimens were stomatitis (any grade, 69.2% and 56.9%, respectively), rash (any grade, 27.7% and 32.3%), diarrhea (any grade, 16.9% and 12.3%), and hyperglycemia (any grade, 9.2% and 11.5%).
The PAM pathway drives breast cancer growth and contributes to endocrine and CDK4/6 inhibitor resistance; however, as most available therapies are indicated only for patients with PI3K-pathway activation, an unmet need remains for those with wild type disease.
“Therapeutic attempts to completely block the PAM pathway have been limited by toxicity,” Hurvitz explained. “Most available therapies target a single component of the pathway and have modest efficacy limited to biomarker selected patient populations.”
Following preliminary clinical activity of gedatolisib in combination with palbociclib and fulvestrant as a therapy in the second line and beyond among patients with HR-positive, HER2-negative advanced breast cancer, the investigators aimed to evaluate the highly potent multitarget PAM inhibitor in this patient population following progression on a CDK4/6 inhibitor and aromatase inhibitor.
In the open-label, randomized phase 3 trial, 392 patients were randomized 1:1:1 to receive either of the following regimens:
Patients treated with fulvestrant monotherapy were allotted the option to cross over to arms A or B at progression.
Pre- and postmenopausal patients were eligible for the trial if they had progression on or after treatment with a CDK4/6 inhibitor and aromatase inhibitor, 2 or more lines of prior endocrine therapy for advanced breast cancer, measurable disease via RECIST v1.1, and a screening result for PIK3CA status. Patients were ineligible if they had type 2 diabetes mellitus with a glycated hemoglobin of greater than 6.4% or type 1 diabetes mellitus; prior therapy with an mTOR, PI3K, or AKT inhibitor; or chemotherapy treatment for their advanced breast cancer.
Patients were stratified by lung/liver metastases, time to progression on their immediate prior therapy, and region.
The co-primary end points were PFS in arm A compared with arm C and PFS in arm B vs arm C. Secondary end points included OS, ORR, safety, and quality of life.
Demographics and baseline characteristics were generally well balanced across treatment arms.
“We are very excited that treatment with gedatolisib combined with fulvestrant with or without palbociclib was well-tolerated by the VIKTORIA-1 patients and that only a few patients discontinued treatment due to an adverse event,” said Igor Gorbatchevsky, MD, chief medical officer of Celcuity, in a press release.2
“This safety profile combined with the 7.3- and 5.4-months incremental improvement in median PFS relative to fulvestrant for the gedatolisib regimens, offer potentially paradigm shifting results for patients with HR-positive, HER2-negative, PIK3CA wild-type advanced breast cancer,” he added.
According to the release, a rolling new drug application (NDA) has been submitted in tandem with the FDA’s Real-Time Oncology Review Program based on data from the PIK3CA wild-type cohort of the phase 3 VIKTORIA-1 trial. The NDA is expected to be completed before the end of 2025, and topline data from the trial is expected in the first half of 2026. The phase 3 VIKTORIA-2 trial (NCT06757634), designed to evaluate gedatolisib in the frontline setting, is ongoing.
How fast we lose teeth in old age has been linked to a person’s risk of dying in a comprehensive new study, emphasizing the importance of good oral health, and suggesting tooth loss could be a key indicator of other serious health…


Researchers have developed a lightweight computer mouse that you wear on your finger like a ring and works for more than a month on a single charge.
Called picoRing, the device weighs just 0.18 ounces (5 grams) and is designed as a discreet,…

While out photographing the dark skies of New Zealand…
This very well received free Steam game just got some brand new content which, surprisingly, is also free. Free stuff for free stuff, you love to see it.
There’s an unquantifiably large amount of free games on Steam. I mean, someone could…