A new artificial intelligence (AI) model using MRI scans of the pituitary gland has demonstrated strong potential in predicting growth hormone deficiency (GHD) among children with short stature. Developed through a multicentre study, the…
Blog
-
With Zelda on vinyl, Nintendo is expanding into the music business
Nintendo is exploring ways to keep its worlds alive not just through new video games, but by expanding its intellectual property into every medium that can hum, glow and now spin at 33⅓ RPM.
The 136-year-old entertainment firm is issuing its…
Continue Reading
-
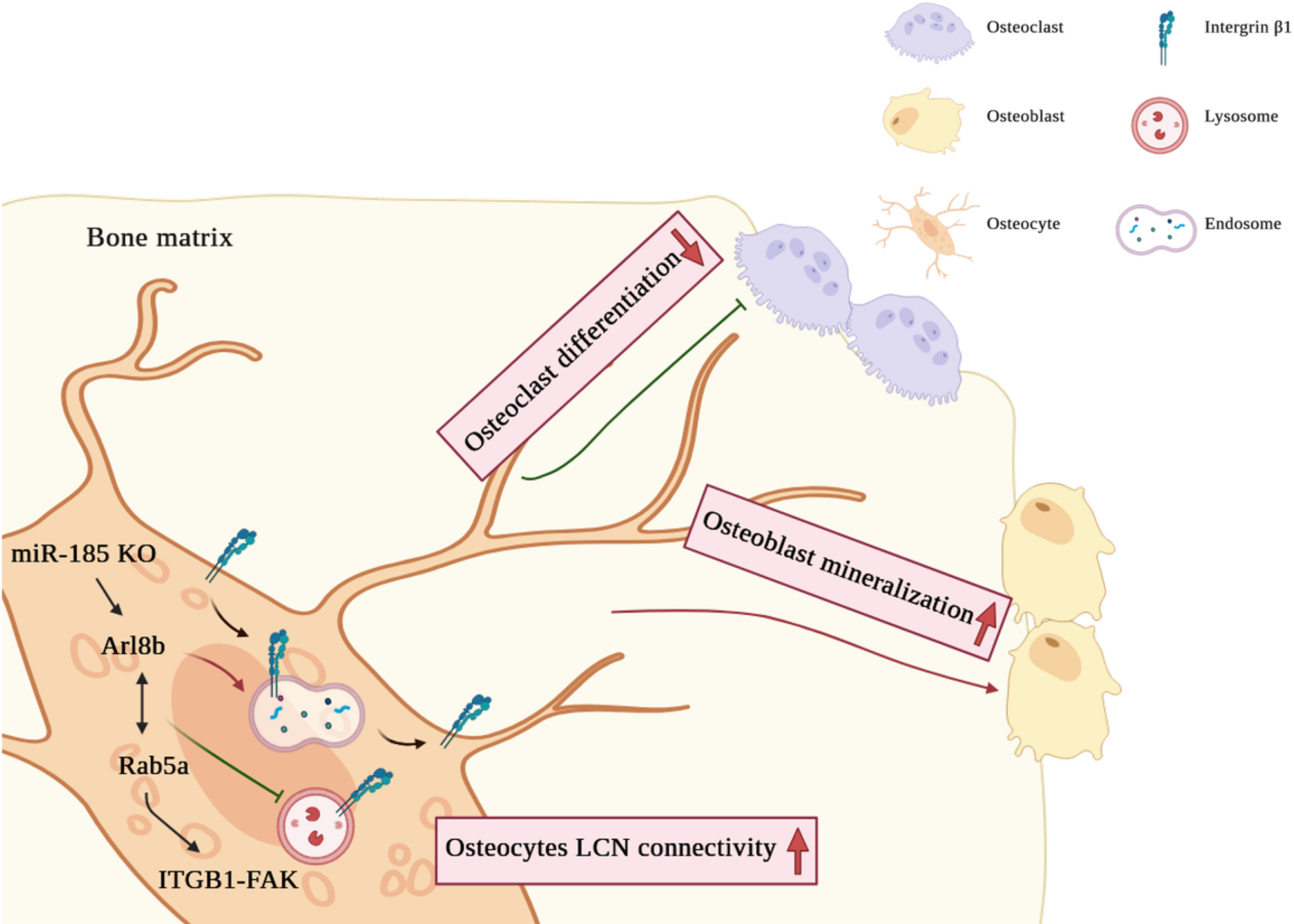
Depletion of mmu-miR-185 enhances osteocyte connectivity and suppresses bone fragility through the interaction between ARL8B and RAB5A | Stem Cell Research & Therapy
Xu H, Ning D, Zhao D, Chen Y, Zhao D, Gu S, Jiang JX, Shang P. Blockage of hemichannels alters gene expression in osteocytes in a high magneto-gravitational environment [J]. Frontiers in bioscience (Landmark edition), 2017, 22: 783–94.
Bouxsein…
Continue Reading
-

Samsung Powers Pearson’s Revibe Smartwatch-based Solution to Help Children and Adults with Focus and Attention Challenges
Children and adults face challenges with focus and attention. This not only makes learning difficult but can seriously impact a person’s ability to plan tasks, complete work and finish assignments. With children, the effects of limited focus can be particularly pronounced, inhibiting their educational, social and emotional development.
Samsung Electronics, a world leader in consumer electronics, and Pearson (FTSE: PSON.L), the world’s lifelong learning company, are collaborating to help children and adults overcome these challenges.
Pearson recently introduced Revibe, an AI-enabled wearable solution delivered via the Samsung Galaxy Watch7, to help individuals build skills in focus, attention and self-regulation.
Revibe tracks on-task behavior, fidgeting, work completion and exercise while providing reminders to stay focused, remember tasks and complete work, which, as part of a healthy lifestyle, may help individuals living with conditions such as ADHD.
By leveraging AI to translate real-time behavioral data into actionable insights, Revibe equips professionals and individual users with data-informed pathways to improve focus in the classroom and beyond.
Combining Pearson’s proprietary, attention-enhancing software with the Galaxy Watch7, including Samsung’s Knox mobile security platform, Revibe is a discreet, real-time tool designed to help users improve concentration and develop stronger self-regulation skills throughout the day. This collaboration reflects a shared commitment to advancing innovation and creating inclusive, accessible solutions that empower individuals of all ages who are navigating focus and attention-related challenges.
Using AI and advanced algorithms to understand Galaxy Watch sensor data, Revibe learns each user’s behavior patterns, including attention span, fidgeting, steps, calories burned and more. Revibe’s software then addresses individuals with focus and attention challenges from multiple angles. Vibrating alerts bring the user back on task and bolster executive function, while on-screen “light bulb moments” provide guidance that won’t disturb others.
Leveraging Samsung’s Freestanding Mode on the Galaxy Watch, Revibe also eliminates smartphone distractions by enabling the Galaxy Watch to operate independently, without a smartphone, for a streamlined user experience. Freestanding Mode is especially important when Revibe is used by children, since most smartwatches are simply an extension of a smartphone, and many schools don’t allow children to carry phones.

The Revibe app offers users, families, educators, and clinicians a user-friendly dashboard that visualizes progress in near real time, which can lead to more customized support in the classroom and elsewhere to help individuals succeed.
“With Revibe, Pearson empowers individuals who experience focus and attention barriers, along with their families and support networks, by helping them build the self-regulation skills they need for success,” said Rich Brancaccio, Senior Director, Pearson, and the Founder of Revibe. “After evaluating multiple wearable solutions, we determined that the Samsung Galaxy Watch was the right device for Revibe, offering the ideal balance of a low-distraction interface, extended battery life1 and secure data collection capabilities to serve the needs of these individuals and help them reach their fullest potential.”

“Samsung Galaxy Watch perfectly fits Revibe’s needs thanks to capabilities such as Samsung’s Knox mobile security management platform, Freestanding Mode, and Kiosk Mode,” said Cherry Drulis, MBA, BSN, RN, Senior Director, Regulated Industry Samsung. “With Knox, Revibe can apply policies to the Galaxy Watch, including software updates to ensure continued compatibility, then detach it from its phone dependency as a freestanding device. Freestanding Mode maintains location tracking so lost devices can be recovered2, while Kiosk Mode keeps Galaxy Watch focused on Revibe’s application, ensuring individuals with focus and attention challenges enjoy easier access with fewer distractions.”
The Samsung and Revibe collaboration will begin with the Samsung Galaxy Watch7 series and is expected to expand to additional Samsung devices. Revibe will offer the solution to clinical professionals across education and healthcare, as well as individual users, parents, and other care teams.
To learn more about Samsung and Pearson’s collaboration, please visit: https://insights.samsung.com/2025/10/29/the-power-of-collaboration or watch the video: https://www.youtube.com/watch?v=ILiCdec7rp4
For more information on the Revibe wearable, please visit: https://www.pearsonassessments.com/campaign/revibe.html
For more information on Samsung Galaxy Watch7, please visit: https://www.samsung.com/us/watches/galaxy-watch7/
Continue Reading
-
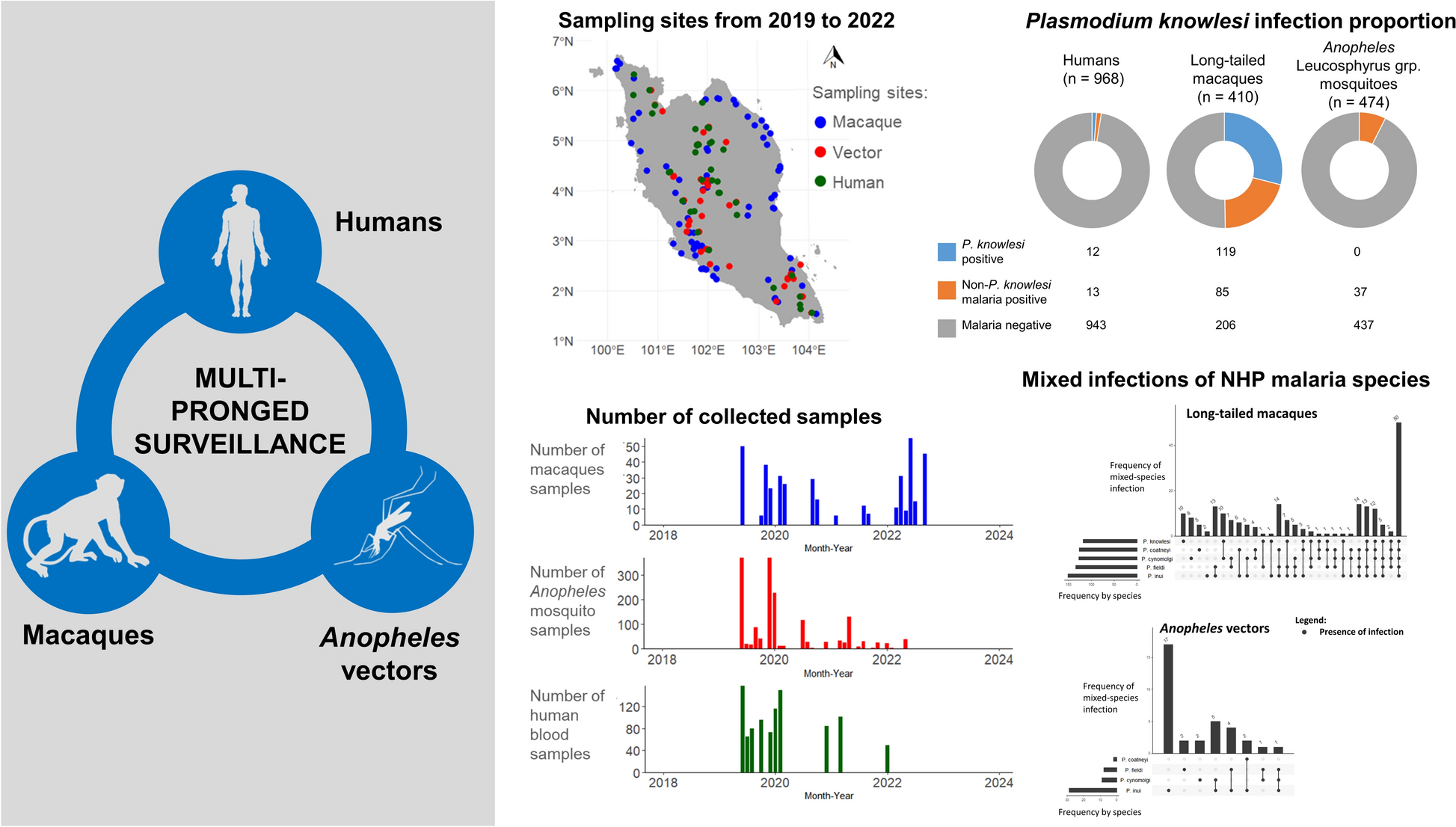
Multi-pronged surveillance to understand the spatiotemporal correlations among macaques, vectors and humans in Plasmodium knowlesi malaria transmission | Parasites & Vectors
World Health Organization. World malaria report 2024. Geneva, Switzerland: World Health Organization; 2024.
Google Scholar
Cuenca PR, Key S, Jumail A, Surendra H, Ferguson HM, Drakeley CJ, et al….
Continue Reading
-

Luke Evans Will Make Broadway Debut in ‘Rocky Horror Picture Show’
Luke Evans will lead the Broadway revival of The Rocky Horror Show this season.
Evans, who appeared as Gaston in Disney’s live action Beauty and the Beast, will make his Broadway debut in the production as Frank-N-Furter. The musical…
Continue Reading
-

Luke Evans to star in ‘The Rocky Horror Show’ on Broadway
The screen and stage star will make his Broadway debut in Roundabout Theatre Company’s revival of the cult classic musical directed by Sam Pinkleton.
The anticipation is over! Roundabout Theatre Company has announced that Luke Evans will star in…
Continue Reading
-
Just a moment…
Just a moment… This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Continue Reading
-
Trump Says South Korea Trade Deal Is Virtually Done – The Wall Street Journal
- Trump Says South Korea Trade Deal Is Virtually Done The Wall Street Journal
- S Korea announces lowering of some tariffs as part of new US trade deal BBC
- Trump and South Korea reach agreement on trade deal details as shutdown impacts widen in US
Continue Reading
-
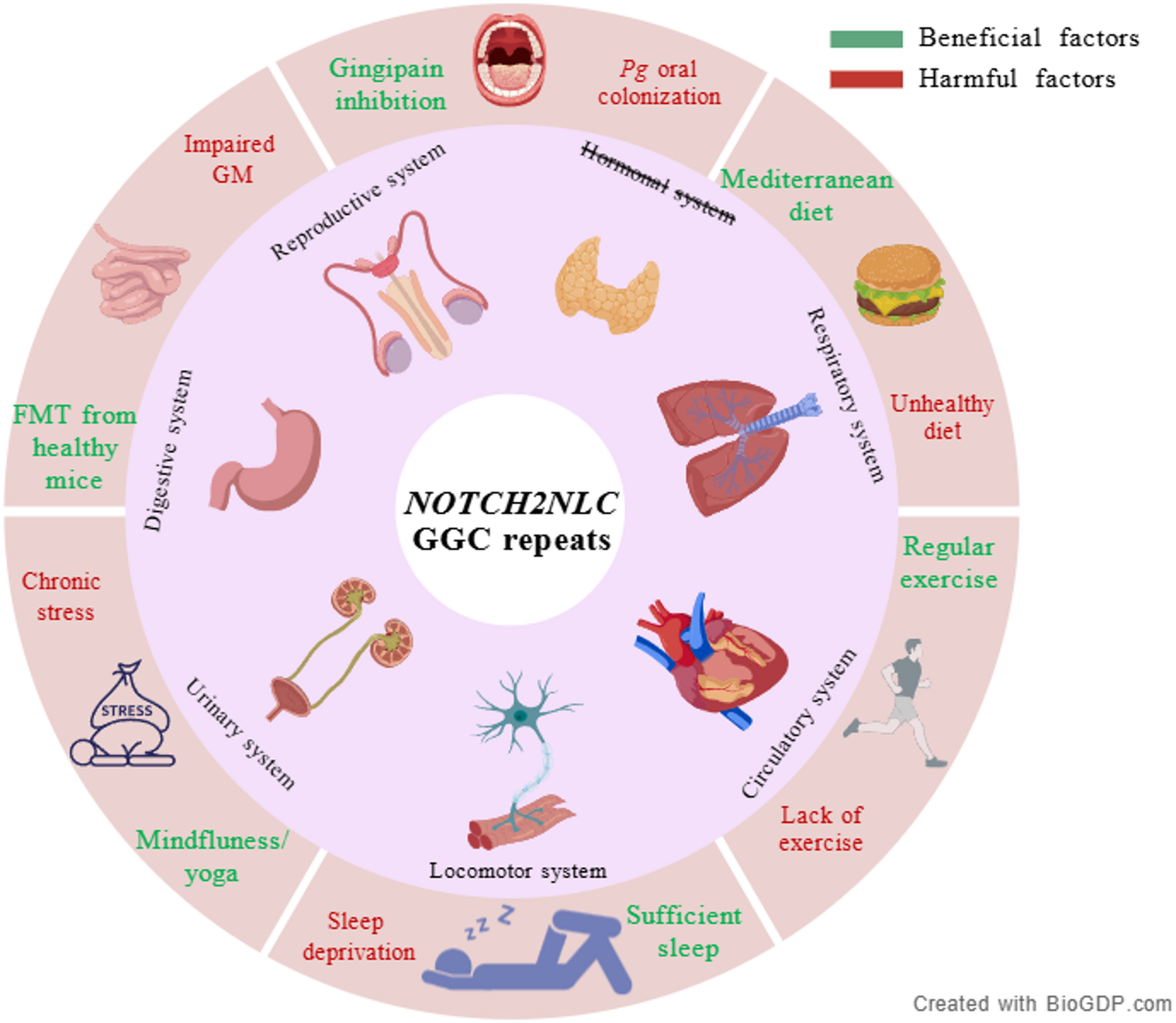
Inflammation in Neuronal Intranuclear Inclusion Disease (NIID): mechanisms, biomarkers, and therapeutic implications | Journal of Neuroinflammation
Sung JH, et al. An unusual degenerative disorder of neurons associated with a novel intranuclear hyaline inclusion (neuronal intranuclear hyaline inclusion disease). A clinicopathological study of a case. J Neuropathol Exp Neurol. 1980;39(2):107–30.
Google Scholar
Sone J, et al. Clinicopathological features of adult-onset neuronal intranuclear inclusion disease. Brain. 2016;139(Pt 12):3170–86.
Google Scholar
Sone J, et al. Long-read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat Genet. 2019;51(8):1215–21.
Google Scholar
Tian Y, et al. Expansion of human-specific GGC repeat in neuronal intranuclear inclusion disease-related disorders. Am J Hum Genet. 2019;105(1):166–76.
Google Scholar
Sun QY, et al. Expansion of GGC repeat in the human-specific NOTCH2NLC gene is associated with essential tremor. Brain. 2020;143(1):222–33.
Google Scholar
Ehrlich ME, Ellerby LM. Neuronal intranuclear inclusion disease: polyglycine protein is the culprit. Neuron. 2021;109(11):1757–60.
Google Scholar
Yu J, et al. CGG repeat expansion in NOTCH2NLC causes mitochondrial dysfunction and progressive neurodegeneration in drosophila model. Proc Natl Acad Sci U S A. 2022;119(41):e2208649119.
Google Scholar
Liu Q, et al. Expression of expanded GGC repeats within NOTCH2NLC causes behavioral deficits and neurodegeneration in a mouse model of neuronal intranuclear inclusion disease. Sci Adv. 2022;8(47):eadd6391.
Google Scholar
Zhong S, et al. Upstream open reading frame with NOTCH2NLC GGC expansion generates polyglycine aggregates and disrupts nucleocytoplasmic transport: implications for polyglycine diseases. Acta Neuropathol. 2021;142(6):1003–23.
Google Scholar
Fan Y, et al. GGC repeat expansion in NOTCH2NLC induces dysfunction in ribosome biogenesis and translation. Brain. 2023;146(8):3373–91.
Google Scholar
Tian Y, et al. Clinical features of NOTCH2NLC-related neuronal intranuclear inclusion disease. J Neurol Neurosurg Psychiatry. 2022;93(12):1289–98.
Google Scholar
Chen H, et al. Re-defining the clinicopathological spectrum of neuronal intranuclear inclusion disease. Ann Clin Transl Neurol. 2020;7(10):1930–41.
Google Scholar
Tai H, et al. Clinical features and classification of neuronal intranuclear inclusion disease. Neurol Genet. 2023;9(2):e200057.
Google Scholar
Jiang S, et al. Generic diagramming platform (GDP): a comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2025;53(D1):D1670–6.
Google Scholar
Pang J, et al. The value of NOTCH2NLC gene detection and skin biopsy in the diagnosis of neuronal intranuclear inclusion disease. Front Neurol. 2021;12:624321.
Google Scholar
Lee HG, et al. Neuroinflammation: an astrocyte perspective. Sci Transl Med. 2023;15(721):eadi7828.
Google Scholar
Bao L et al. Immune system involvement in neuronal intranuclear inclusion disease. Neuropathol Appl Neurobiol, 2024;50(2):e12976.
Mori K, et al. Imaging findings and pathological correlations of subacute encephalopathy with neuronal intranuclear inclusion disease-case report. Radiol Case Rep. 2022;17(12):4481–6.
Google Scholar
Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci. 2017;20(2):136–44.
Google Scholar
Yoshii D, et al. An autopsy case of adult-onset neuronal intranuclear inclusion disease with perivascular preservation in cerebral white matter. Neuropathology. 2021;42(1):66–73.
Google Scholar
Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503.
Google Scholar
Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117(14):3720–32.
Google Scholar
Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295.
Google Scholar
Lu X, Hong D. Neuronal intranuclear inclusion disease: recognition and update. J Neural Transm. 2021;128(3):295–303.
Google Scholar
Bao L, et al. Utility of labial salivary gland biopsy in the histological diagnosis of neuronal intranuclear inclusion disease. Eur J Neurol. 2024;31(1):e16102.
Google Scholar
Zhong S, et al. Spatial and Temporal distribution of white matter lesions in NOTCH2NLC-Related neuronal intranuclear inclusion disease. Neurology. 2025;104(4):e213360.
Google Scholar
Du N et al. Value of neutrophil-to-lymphocyte ratio in neuronal intranuclear inclusion disease. Heliyon, 2024;10(6):e27953.
Yan Y, et al. The clinical characteristics of neuronal intranuclear inclusion disease and its relation with inflammation. Neurol Sci. 2023;44(9):3189–97.
Google Scholar
Shen Y et al. uN2CpolyG-mediated p65 nuclear sequestration suppresses the NF-κB-NLRP3 pathway in neuronal intranuclear inclusion disease. Cell Communication Signal, 2025;23(1):68.
Tateishi J, et al. Intranuclear inclusions in muscle, nervous tissue, and adrenal gland. Acta Neuropathol. 1984;63(1):24–32.
Google Scholar
Platero JL et al. The impact of coconut oil and Epigallocatechin gallate on the levels of IL-6, anxiety and disability in multiple sclerosis patients. Nutrients, 2020;12(2):305.
Casali BT, et al. Omega-3 fatty acids augment the actions of nuclear receptor agonists in a mouse model of alzheimer’s disease. J Neurosci. 2015;35(24):9173–81.
Google Scholar
Chauhan A, et al. Phytochemicals targeting NF-κB signaling: potential anti-cancer interventions. J Pharm Anal. 2022;12(3):394–405.
Google Scholar
Shabab T, et al. Neuroinflammation pathways: a general review. Int J Neurosci. 2017;127(7):624–33.
Google Scholar
Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651.
Google Scholar
Gleeson M, et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–15.
Google Scholar
Pedersen BK. Adolph distinguished lecture: muscle as an endocrine organ: IL-6 and other myokines. J Appl Physiol (1985). 2009;107(4):1006–14. Edward F.
Google Scholar
Steensberg A, et al. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285(2):E433–7.
Google Scholar
Ma Q. Beneficial effects of moderate voluntary physical exercise and its biological mechanisms on brain health. Neurosci Bull. 2008;24(4):265–70.
Google Scholar
Adlard PA, et al. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25(17):4217–21.
Google Scholar
Periasamy S, et al. Sleep deprivation-induced multi-organ injury: role of oxidative stress and inflammation. Excli J. 2015;14:672–83.
Google Scholar
Shearer WT, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107(1):165–70.
Google Scholar
Ooms S, et al. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 2014;71(8):971–7.
Google Scholar
Reiter RJ, et al. Brain washing and neural health: role of age, sleep, and the cerebrospinal fluid melatonin rhythm. Cell Mol Life Sci. 2023;80(4):88.
Google Scholar
Zhao HY, et al. Chronic sleep restriction induces cognitive deficits and cortical Beta-Amyloid deposition in mice via BACE1-Antisense activation. CNS Neurosci Ther. 2017;23(3):233–40.
Google Scholar
Zhang Y, et al. Quercetin ameliorates memory impairment by inhibiting abnormal microglial activation in a mouse model of Paradoxical sleep deprivation. Biochem Biophys Res Commun. 2022;632:10–6.
Google Scholar
Irwin MR, et al. Tai Chi compared with cognitive behavioral therapy and the reversal of systemic, cellular and genomic markers of inflammation in breast cancer survivors with insomnia: A randomized clinical trial. Brain Behav Immun. 2024;120:159–66.
Google Scholar
Dutcher JM, et al. Smartphone mindfulness meditation training reduces Pro-inflammatory gene expression in stressed adults: A randomized controlled trial. Brain Behav Immun. 2022;103:171–7.
Google Scholar
Walsh E, Eisenlohr-Moul T, Baer R. Brief mindfulness training reduces salivary IL-6 and TNF-α in young women with depressive symptomatology. J Consult Clin Psychol. 2016;84(10):887–97.
Google Scholar
Patel DI, et al. Therapeutic yoga reduces pro-tumorigenic cytokines in cancer survivors. Support Care Cancer. 2022;31(1):33.
Google Scholar
Streeter CC, et al. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses. 2012;78(5):571–9.
Google Scholar
Ozturk FO, Tezel A. Effect of laughter yoga on mental symptoms and salivary cortisol levels in first-year nursing students: A randomized controlled trial. Int J Nurs Pract. 2021;27(2):e12924.
Google Scholar
Nair RG, Vasudev MM, Mavathur R. Role of yoga and its plausible mechanism in the mitigation of DNA damage in Type-2 diabetes: A randomized clinical trial. Ann Behav Med. 2022;56(3):235–44.
Google Scholar
Chen SJ, et al. Association of fecal and plasma levels of Short-Chain fatty acids with gut microbiota and clinical severity in patients with Parkinson disease. Neurology. 2022;98(8):e848–58.
Google Scholar
Chakraborty P, Gamage H, Laird AS. Butyrate as a potential therapeutic agent for neurodegenerative disorders. Neurochem Int. 2024;176:105745.
Google Scholar
Li N, et al. Prebiotic inulin controls Th17 cells mediated central nervous system autoimmunity through modulating the gut microbiota and short chain fatty acids. Gut Microbes. 2024;16(1):2402547.
Google Scholar
Zhang Y, et al. Transmission of alzheimer’s disease-associated microbiota dysbiosis and its impact on cognitive function: evidence from mice and patients. Mol Psychiatry. 2023;28(10):4421–37.
Google Scholar
Kim MS, et al. Transfer of a healthy microbiota reduces amyloid and Tau pathology in an alzheimer’s disease animal model. Gut. 2020;69(2):283–94.
Google Scholar
Beydoun MA, et al. Clinical and bacterial markers of periodontitis and their association with incident All-Cause and alzheimer’s disease dementia in a large National survey. J Alzheimers Dis. 2020;75(1):157–72.
Google Scholar
Ilievski V, et al. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLoS ONE. 2018;13(10):e0204941.
Google Scholar
Wang RP, et al. IL-1β and TNF-α play an important role in modulating the risk of periodontitis and alzheimer’s disease. J Neuroinflammation. 2023;20(1):71.
Google Scholar
Dominy SS, et al. Porphyromonas gingivalis in alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5(1):eaau3333.
Google Scholar
Brettschneider J, et al. Microglial activation correlates with disease progression and upper motor neuron clinical symptoms in amyotrophic lateral sclerosis. PLoS ONE. 2012;7(6):e39216.
Google Scholar
Graves MC, et al. Inflammation in amyotrophic lateral sclerosis spinal cord and brain is mediated by activated macrophages, mast cells and T cells. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5(4):213–9.
Google Scholar
Henkel JS, et al. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2004;55(2):221–35.
Google Scholar
Liu YH, et al. Neuronal intranuclear inclusion disease in patients with adult-onset non-vascular leukoencephalopathy. Brain. 2022;145(9):3010–21.
Google Scholar
Chen L, et al. Teaching neuroimages: the zigzag edging sign of adult-onset neuronal intranuclear inclusion disease. Neurology. 2019;92(19):e2295-6.
Google Scholar
Fragoso DC, et al. Imaging of Creutzfeldt-Jakob disease: imaging patterns and their differential diagnosis. Radiographics. 2017;37(1):234–57.
Google Scholar
Muttikkal TJ, Wintermark M. MRI patterns of global hypoxic-ischemic injury in adults. J Neuroradiol. 2013;40(3):164–71.
Google Scholar
Hasebe M, et al. Hypoglycemic encephalopathy. QJM. 2022;115(7):478–9.
Google Scholar
Zhang Z, et al. MRI features of neuronal intranuclear inclusion disease, combining visual and quantitative imaging investigations. J Neuroradiol. 2024;51(3):274–80.
Google Scholar
Okamura S, et al. A case of neuronal intranuclear inclusion disease with recurrent vomiting and without apparent DWI abnormality for the first seven years. Heliyon. 2020;6(8):e04675.
Google Scholar
Liu Y, et al. Clinical and mechanism advances of neuronal intranuclear inclusion disease. Front Aging Neurosci. 2022;14:934725.
Google Scholar
Liang H, et al. Clinical and pathological features in adult-onset NIID patients with cortical enhancement. J Neurol. 2020;267(11):3187–98.
Google Scholar
Shen Y, et al. Encephalitis-like episodes with cortical edema and enhancement in patients with neuronal intranuclear inclusion disease. Neurol Sci. 2024;45(9):4501–11.
Google Scholar
Jacobs AH, Tavitian B. Noninvasive molecular imaging of neuroinflammation. J Cereb Blood Flow Metab. 2012;32(7):1393–415.
Google Scholar
Corica F et al. PET imaging of Neuro-Inflammation with tracers targeting the translocator protein (TSPO), a systematic review: from bench to bedside. Diagnostics (Basel), 2023;13(6):1029.
Banati RB. Visualising microglial activation in vivo. Glia. 2002;40(2):206–17.
Google Scholar
Turner MR, et al. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11 C](R)-PK11195 positron emission tomography study. Neurobiol Dis. 2004;15(3):601–9.
Google Scholar
Tondo G, et al. (11) C-PK11195 PET-based molecular study of microglia activation in SOD1 amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2020;7(9):1513–23.
Google Scholar
Malpetti M, et al. Microglial activation in the frontal cortex predicts cognitive decline in frontotemporal dementia. Brain. 2023;146(8):3221–31.
Google Scholar
Zhong S, et al. Microglia contribute to polyG-dependent neurodegeneration in neuronal intranuclear inclusion disease. Acta Neuropathol. 2024;148(1):21.
Google Scholar
Hutton BF. The origins of SPECT and SPECT/CT. Eur J Nucl Med Mol Imaging. 2014;41(Suppl 1):S3-16.
Google Scholar
Benadiba M, et al. New molecular targets for PET and SPECT imaging in neurodegenerative diseases. Braz J Psychiatry. 2012;34(Suppl 2):S125–36.
Google Scholar
Kimachi T, et al. Reversible encephalopathy with focal brain edema in patients with neuronal intranuclear inclusion disease. Neurology and Clinical Neuroscience. 2017;5(6):198–200.
Google Scholar
Fujita K, et al. Neurologic attack and dynamic perfusion abnormality in neuronal intranuclear inclusion disease. Neurol Clin Pract. 2017;7(6):e39-42.
Google Scholar
Pan Y, et al. Expression of expanded GGC repeats within NOTCH2NLC causes cardiac dysfunction in mouse models. Cell Biosci. 2023;13(1):157.
Google Scholar
Heslegrave A, et al. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer’s disease. Mol Neurodegener. 2016;11:3.
Google Scholar
Craig-Schapiro R, et al. YKL-40: a novel prognostic fluid biomarker for preclinical alzheimer’s disease. Biol Psychiatry. 2010;68(10):903–12.
Google Scholar
Crols R, et al. Increased GFAP levels in CSF as a marker of organicity in patients with Alzheimer’s disease and other types of irreversible chronic organic brain syndrome. J Neurol. 1986;233(3):157–60.
Google Scholar
Andrés-Benito P, et al. Differential astrocyte and oligodendrocyte vulnerability in murine Creutzfeldt-Jakob disease. Prion. 2021;15(1):112–20.
Google Scholar
Mussbacher M, et al. NF-κB in monocytes and macrophages – an inflammatory master regulator in multitalented immune cells. Front Immunol. 2023;14:1134661.
Google Scholar
Biasizzo M, Kopitar-Jerala N. Interplay between NLRP3 inflammasome and autophagy. Front Immunol. 2020;11:591803.
Google Scholar
Deng Q, et al. Pseudomonas aeruginosa triggers macrophage autophagy to escape intracellular killing by activation of the NLRP3 inflammasome. Infect Immun. 2016;84(1):56–66.
Google Scholar
Geissmann F, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–61.
Google Scholar
Guilliams M, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14(8):571–8.
Google Scholar
Prinz M, Jung S, Priller J. Microglia Biology: One Century Evol Concepts Cell. 2019;179(2):292–311.
Google Scholar
Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18(4):225–42.
Google Scholar
Liddelow SA, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–7.
Google Scholar
Keren-Shaul H, et al. A unique microglia type associated with restricting development of alzheimer’s disease. Cell. 2017;169(7):1276–e129017.
Google Scholar
Mathys H, et al. Temporal tracking of microglia activation in neurodegeneration at single-cell resolution. Cell Rep. 2017;21(2):366–80.
Google Scholar
Zhou Y, et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat Med. 2020;26(1):131–42.
Google Scholar
Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44(3):439–49.
Google Scholar
Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514.
Google Scholar
Merad M, et al. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604.
Google Scholar
Sun R, Jiang H. Border-associated macrophages in the central nervous system. J Neuroinflammation. 2024;21(1):67.
Google Scholar
Goldmann T, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016;17(7):797–805.
Google Scholar
Van Hove H, et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci. 2019;22(6):1021–35.
Google Scholar
Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118(1):103–13.
Google Scholar
Faraco G, et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest. 2016;126(12):4674–89.
Google Scholar
Rua R, et al. Infection drives meningeal engraftment by inflammatory monocytes that impairs CNS immunity. Nat Immunol. 2019;20(4):407–19.
Google Scholar
Dani N, et al. A cellular and Spatial map of the choroid plexus across brain ventricles and ages. Cell. 2021;184(11):3056–e307421.
Google Scholar
Mrdjen D, et al. High-Dimensional Single-Cell mapping of central nervous system immune cells reveals distinct myeloid subsets in Health, Aging, and disease. Immunity. 2018;48(2):380–95. .e6.
Google Scholar
Park L, et al. Brain perivascular macrophages initiate the neurovascular dysfunction of Alzheimer Aβ peptides. Circ Res. 2017;121(3):258–69.
Google Scholar
Schläger C, et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature. 2016;530(7590):349–53.
Google Scholar
Herz J, et al. Role of neutrophils in exacerbation of brain injury after focal cerebral ischemia in hyperlipidemic mice. Stroke. 2015;46(10):2916–25.
Google Scholar
Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–60.
Google Scholar
Greenberg SM, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165–74.
Google Scholar
Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry. 2012;83(2):124–37.
Google Scholar
Liao YC, et al. NOTCH2NLC GGC repeat expansion in patients with vascular leukoencephalopathy. Stroke. 2023;54(5):1236–45.
Google Scholar
Bao L, et al. GGC repeat expansions in NOTCH2NLC cause uN2CpolyG cerebral amyloid angiopathy. Brain. 2025;148(2):467–79.
Google Scholar
Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J Cell Mol Med. 2009;13(9a):2911–25.
Google Scholar
Shan Y, et al. The glucagon-like peptide-1 receptor agonist reduces inflammation and blood-brain barrier breakdown in an astrocyte-dependent manner in experimental stroke. J Neuroinflammation. 2019;16(1):242.
Google Scholar
Orihara A, et al. Acute reversible encephalopathy with neuronal intranuclear inclusion disease diagnosed by a brain biopsy: inferring the mechanism of encephalopathy from radiological and histological findings. Intern Med. 2023;62(12):1821–5.
Google Scholar
Li YY, et al. Interactions between beta-Amyloid and pericytes in Alzheimer’s disease. Front Biosci (Landmark Ed). 2024;29(4):136.
Google Scholar
Huang X, Hussain B, Chang J. Peripheral inflammation and blood-brain barrier disruption: effects and mechanisms. CNS Neurosci Ther. 2021;27(1):36–47.
Google Scholar
Rahimifard M, et al. Targeting the TLR4 signaling pathway by polyphenols: a novel therapeutic strategy for neuroinflammation. Ageing Res Rev. 2017;36:11–9.
Google Scholar
Zhang M, et al. Blockage of VEGF function by bevacizumab alleviates early-stage cerebrovascular dysfunction and improves cognitive function in a mouse model of Alzheimer’s disease. Transl Neurodegener. 2024;13(1):1.
Google Scholar
Wild CP. Complementing the genome with an exposome: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847–50.
Google Scholar
Doroszkiewicz J et al. Common and trace metals in alzheimer’s and parkinson’s diseases. Int J Mol Sci, 2023;24(21):15721.
Gu Y, et al. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer’s disease. J Alzheimers Dis. 2010;22(2):483–92.
Google Scholar
Flanagan E, et al. Nutrition and the ageing brain: moving towards clinical applications. Ageing Res Rev. 2020;62:101079.
Google Scholar
Gelpi E, et al. Neuronal intranuclear (hyaline) inclusion disease and fragile X-associated tremor/ataxia syndrome: a morphological and molecular dilemma. Brain. 2017;140(8):e51.
Google Scholar
Tolar M et al. Neurotoxic soluble amyloid oligomers drive alzheimer’s pathogenesis and represent a clinically validated target for slowing disease progression. Int J Mol Sci, 2021;22(12):6355.
Habashi M, et al. Early diagnosis and treatment of alzheimer’s disease by targeting toxic soluble Aβ oligomers. Proc Natl Acad Sci U S A. 2022;119(49):e2210766119.
Google Scholar
Wang ZX, et al. The essential role of soluble Aβ oligomers in alzheimer’s disease. Mol Neurobiol. 2016;53(3):1905–24.
Google Scholar
Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99(3):1325–80.
Google Scholar
Su WJ, et al. Antidiabetic drug glyburide modulates depressive-like behavior comorbid with insulin resistance. J Neuroinflammation. 2017;14(1):210.
Google Scholar
Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10(10):717–25.
Google Scholar
Zhang J, et al. Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice. J Neuroinflammation. 2018;15(1):37.
Google Scholar
Nie R, et al. Porphyromonas gingivalis infection induces Amyloid-β accumulation in Monocytes/Macrophages. J Alzheimers Dis. 2019;72(2):479–94.
Google Scholar
Kemaladewi DU, et al. Correction of a splicing defect in a mouse model of congenital muscular dystrophy type 1A using a homology-directed-repair-independent mechanism. Nat Med. 2017;23(8):984–9.
Google Scholar
Sarkar S, et al. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded Huntingtin and related proteinopathies. Cell Death Differ. 2009;16(1):46–56.
Google Scholar
Grima JC, et al. Mutant Huntingtin disrupts the nuclear pore complex. Neuron. 2017;94(1):93–e1076.
Google Scholar
Continue Reading