New research suggests that those diagnosed with autism in late childhood, adolescence, or young adulthood are more likely to have a different type than those diagnosed in early childhood.
“We found that, on average, individuals diagnosed…

New research suggests that those diagnosed with autism in late childhood, adolescence, or young adulthood are more likely to have a different type than those diagnosed in early childhood.
“We found that, on average, individuals diagnosed…

SpaceX has completed its eleventh integrated flight test of Starship, marking another leap forward in developing the world’s most powerful and fully reusable launch system.
Launched from Starbase, Texas, the flight saw both the Super Heavy…
TOKYO, Japan, October 15, 2025 – Honda Motor Co., Ltd. today announced that it has decided to make an additional investment in Helm.ai, a California-based startup, that has key strengths in AI technologies advanced through unsupervised learning*1. As Helm.ai raises additional funds for its continued growth, Honda will make an additional investment to further enhance its development of next-generation end-to-end (E2E) autonomous driving (AD) and advanced driver-assistance systems (ADAS).
Helm.ai is an AI software startup established in November 2016, and Honda and Helm.ai have been working in collaboration since 2019 through Honda Xcelerator*2, a global open innovation program of Honda. Through the initial investment Honda made in 2022, the two companies have strengthened collaboration and accelerated research and development of unique solutions that integrate advanced AI technologies of Helm.ai and Honda technologies.
Moreover, in July 2025, the two companies signed a multi-year joint development agreement for the purpose of enhancing the development of next-generation AD/ADAS based on the E2E AI architecture that controls everything from environmental perception to decision-making and vehicle actuation.
The additional investment announced today will further strengthen the relationship between the two companies under this overarching direction of the joint development agreement. By leveraging the original Deep Teaching™*3 technology and generative AI of Helm.ai, Honda will accelerate its development of next-generation ADAS which will provide high-level driver assistance, including vehicle operations such as acceleration and steering, throughout the entire route to the destination, whether on expressways or surface roads. Honda is aiming to apply this new, next-generation ADAS to a broad range of key EV and HEV models Honda will launch in North America and Japan around 2027.
Going forward, Honda will continue to accelerate the development of its original next-generation AD/ADAS based on cutting-edge AI technologies including E2E AI, and swiftly deliver highly reliable autonomous driving technologies to customers around the world, which will accelerate Honda initiative to achieve zero fatalities from traffic collisions involving Honda motorcycles and automobiles.

Thus far in Season 9, there have been 41 contracts awarded, meaning that adding five more athletes to the roster this evening would equal the series record for contracts awarded in a season, and if six or more pique White’s interest, a new…

SHANGHAI, CHINA – AUGUST 14, 2025 – Tourists are visiting the Bund in Shanghai, China on August 14, 2025.
Cfoto | Future Publishing | Getty Images
Asia-Pacific markets opened higher Wednesday, breaking ranks with Wall Street’s declines after U.S. and China exchanged blows in a renewed trade feud.
U.S. President Donald Trump on Tuesday stateside criticized China for not buying soybeans, calling it an “an economically hostile act.” He also threatened “retribution” such as a cooking oil embargo.
“Volatility remains elevated, and the best explanation is the strained relationship between the U.S. and China,” Veteran investor Louis Navellier wrote in a note published Wednesday.
Japan’s benchmark Nikkei 225 index rose 0.3%, while the Topix added 0.75%. South Korea’s Kospi jumped 0.8%, while the small-cap Kosdaq added 0.83%.
Australia’s ASX/S&P 200 was up 0.93%.
Hong Kong’s Hang Seng Index was set to open higher, with its futures contract trading at 25,763, against the index’s previous close of 25,441.35.
Investors will be keeping an eye on China’s inflation data for September coming out later in the morning.
Overnight in the U.S., the S&P 500 closed down 0.2% to 6,644.31 in a wild day that saw the benchmark fall as much as 1.5% and gain 0.4% at its highs.
The Nasdaq Composite was off by 0.8% to 22,521.70, although at one point it had fallen as much as 2.1%.The Dow Jones Industrial average closed up 0.4%, or 202.88 points, to 46,270.46 after gaining nearly 1% at one point.
Federal Reserve Chair Jerome Powell on Tuesday suggested the central bank is nearing a point where it will stop reducing the size of its bond holdings, and provided a few hints that more interest rate cuts are in the cards.
— CNBC’s Liz Napolitano and Fred Imbert contributed to this report.

A marketing authorization application (MAA) for the use of relacorilant in patients with platinum-resistant ovarian cancer has been submitted to the European Medicines Agency, according to an announcement from Corcept Therapeutics Incorporated.1
The submission was supported by findings from the
“Our MAA submission brings us a step closer to our goal of delivering relacorilant to patients with platinum-resistant ovarian cancer,” Joseph Belanoff, MD, chief executive officer of Corcept Therapeutics, stated in a news release. “Better treatment options are urgently needed. Relacorilant has the potential to redefine how platinum-resistant ovarian cancer is treated.”
The phase 3 trial enrolled patients (n = 381) with epithelial ovarian, primary peritoneal or fallopian tube cancer who experienced progression within 6 months after their last dose of platinum therapy.2 Patients had an ECOG performance status of 0 or 1, had previously received 1 to 3 lines of therapy, and had prior exposure to bevacizumab (Avastin).
Participants were randomized 1:1 to receive 150 mg of relacorilant plus 80 mg/m2 of nab-paclitaxel or 100 mg/m2 (n = 188) of nab-paclitaxel alone (n = 193). Treatment continued until disease progression or intolerable toxicity. Stratification factors included prior lines of therapy (1 vs >1) and region (North America vs Europe vs Korea, Australia, and Latin America).
The dual primary end points of the study were PFS by blinded independent central review and RECIST 1.1 criteria and OS. Secondary end points included investigator-assessed PFS, objective response rate (ORR), duration of response, clinical benefit rate (CBR), response by CA-125 Gynecologic Cancer Intergroup (GCIG) criteria, combined response by GCIG and RECIST criteria, and safety.
The median patient age was 61 years (range, 26-85) in the relacorilant arm and 62 years (range, 33-86) in the nab-paclitaxel–alone arm. Most patients were White (72.3% vs 69.9%), and slightly more than half were from Europe (56.9% vs 56.5%). About one-third had an ECOG performance status of 1 or 2 (28.2% vs 32.6%), and around 12% had BRCA1/2 mutations (12.2% vs 12.4%). In the experimental arm, 8.0%, 48.9%, and 43.1% of patients received 1, 2, or 3 prior lines of therapy, respectively; in the control arm, these respective rates were 9.3%, 46.1%, and 44.6%. In the experimental arm, 6.9% of patients were primary platinum refractory, 35.6% had received at least 1 prior line of therapy in the platinum-resistant setting, and 4.3% had prior taxane exposure in the platinum-resistant setting; in the control arm, these rates were 6.7%, 42.5%, and 3.6%. Prior therapies received in the combination and monotherapy arms were bevacizumab (100%; 100%), taxanes (99.5%; 99.5%), pegylated liposomal doxorubicin (64.4%; 64.8%), and PARP inhibition (60.6%; 62.2%).
The median PFS with relacorilant plus nab-paclitaxel was 6.54 months (95% CI, 5.55-7.43) vs 5.52 months (95% CI, 3.94-5.88) with nab-paclitaxel alone, translating to a 30% reduction in the risk of disease progression or death (HR, 0.70; 95% CI, 0.54-0.91; P = .0076). The hazard ratio for PFS per investigator assessment was 0.71 (P = .0030). The 6-month PFS rates in the respective arms were 52% and 42%; the 12-month PFS rates were 25% and 13%.
At the time of the interim analysis, which had a data maturity of 50%, the addition of relacorilant to nab-paclitaxel was also found to improve OS over nab-paclitaxel alone, at a median of 15.97 months (95% CI, 13.47-not reached) and 11.50 months (95% CI, 10.02-13.57), respectively (HR, 0.69; 95% CI, 0.52-0.92; nominal P = .0121). The 12-month OS rates in the respective arms were 60% and 49%.
The relacorilant combination elicited an ORR of 36.9% vs 30.1% with nab-paclitaxel monotherapy, translating to a 6.8% improvement (P = .17). The CBRs in the respective arms were 51.1% and 38.9%, translating to a 12.2% improvement (P = .016).
In terms of safety, ascites was found to be less common in those given relacorilant vs not, with unadjusted incidence rates of 5% and 11%, respectively, for all-grade ascites; for grade 3 or higher, the rates were 3% and 5%.
Treatment-emergent adverse effects (TEAEs) occurred in all patients who received the combination (n = 188) vs 99.5% of those who received the monotherapy (n = 190); they were grade 3 or higher for 74.5% and 59.5% of patients, respectively. Serious adverse effects (AEs) were reported in 35.1% of those in the combination arm and 23.7% of those in the monotherapy arm. AEs that resulted in treatment discontinuation for more than 2 patients were intestinal obstruction and paresthesia. No fatal AEs were tied to relacorilant.
In a past interview with OncLive®,
YouTube has announced a raft of new updates, including a UI refresh, expanded voice replies, expanded access to courses, an update on fixable violations, and more.
First off, YouTube’s rolling…

If you purchase an independently reviewed product or service through a link on our website, The Hollywood Reporter may receive an affiliate commission.
A new true crime drama is about to hit streaming. Based on what’s commonly referred to…
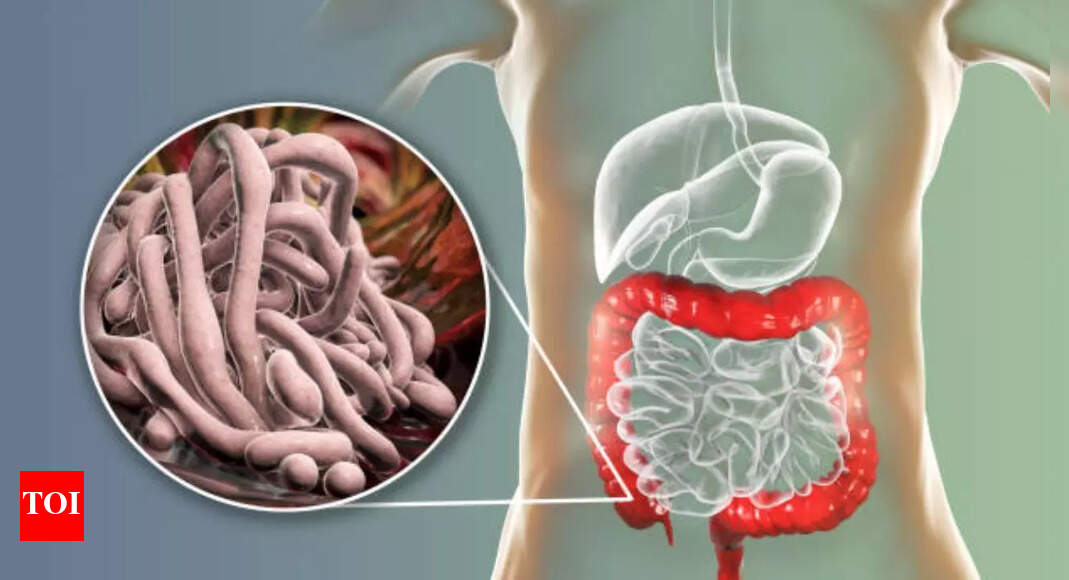
Parasites in the intestines. Sounds alarming right? If a gastroenterologist’s words are to go by then it is. According to Dr Joseph Salhab, a gastroenterologist with a following of 1.5M on Instagram, you could be…

Carrie Preston said Julia Roberts was “mean” to her on the set of 2009’s Duplicity, but she had a good reason.
During a recent interview on Jesse Tyler Ferguson’s Dinner’s on Me podcast, the actress recounted her experience…