The fight against malaria has stalled after two decades of progress, with climate change and population growth among factors threatening a resurgence of the potentially fatal disease, campaigners said Tuesday.
Insufficient funding for…
The fight against malaria has stalled after two decades of progress, with climate change and population growth among factors threatening a resurgence of the potentially fatal disease, campaigners said Tuesday.
Insufficient funding for…
This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!

GSK plc (LSE/NYSE: GSK) today announced the US Food and Drug Administration (FDA) has approved Blenrep (belantamab mafodotin-blmf) in combination with bortezomib and dexamethasone (BVd) for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least two prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory (IMID) agent.
The Blenrep approval is supported by data from the pivotal DREAMM-7 phase III trial. In patients who had two or more prior lines of therapy (3L+), including a PI and an IMID, Blenrep in combination demonstrated a clinically meaningful 51% reduction in the risk of death [HR 0.49, 95% confidence interval (CI): 0.32-0.76] and a tripled median progression-free survival (PFS) of 31.3 months [95% CI: 23.5-NR)] versus 10.4 months [95% CI: 7.0-13.4] for a daratumumab-based triplet (DVd) [HR 0.31, 95% CI: 0.21-0.47]. The safety and tolerability profiles of the Blenrep combination were broadly consistent with the known profiles of the individual agents.2
Tony Wood, Chief Scientific Officer, GSK, said: “Today’s FDA approval of Blenrep is another significant milestone, providing potential for superior efficacy, including overall survival, to US patients. There is an urgent need for new and novel therapies, as nearly all patients with multiple myeloma experience relapse and re-treating with the same mechanism of action often leads to suboptimal outcomes. As the only anti-BCMA agent that can be administered across healthcare settings, including in community centres where 70% of patients receive care, Blenrep fulfils a major patient need. We believe Blenrep can redefine treatment for patients with multiple myeloma in all parts of the world, and we are accelerating its development in earlier lines of therapy to support its use across all stages of this difficult-to-treat cancer.”
Working closely with the FDA, Blenrep is available through a new, streamlined Risk Evaluation and Mitigation Strategy (REMS). The new REMS supports appropriate use and patient safety while reducing administrative burden through simplified patient forms, removal of duplicative checklists and efficient communication between HCPs and either optometrists or ophthalmologists monitoring eye care. GSK will also offer Together with GSK, an optional patient support programme available to all US patients prescribed Blenrep.
Data from the DREAMM (DRiving Excellence in Approaches to Multiple Myeloma) clinical trial programme will be submitted to the National Comprehensive Cancer Network (NCCN) guidelines this year. Recent results from the DREAMM studies, alongside emerging real-world evidence, provide a growing body of data for Blenrep.5,6
Sagar Lonial, MD, Chief Medical Officer, Winship Cancer Institute of Emory University in Atlanta, Georgia, Chair of Emory Department of Hematology and Medical Oncology, said: “With the approval of Blenrep, we now have a community-accessible BCMA-targeting agent with the potential to improve outcomes for patients following two or more prior lines of treatment, where options are limited. This approval marks an important advance in the US relapsed/refractory treatment landscape.”
Michael Andreini, President and Chief Executive Officer of the Multiple Myeloma Research Foundation and the Multiple Myeloma Research Consortium, said: “The reality for most patients with multiple myeloma is a relentless cycle of remission and relapse, as their disease becomes refractory to treatments. Patients urgently need more effective treatment options that can offer more quality time with their loved ones. We see the potential for Blenrep in combination to help patients achieve this.”
GSK is advancing the DREAMM clinical programme to demonstrate Blenrep’s potential benefit in earlier lines of treatment. Follow-up continues for overall survival (OS) in both DREAMM-7 and DREAMM-8 with data expected in early 2028, including in patients who have received only one prior line of therapy. DREAMM-10, a phase III trial in newly diagnosed transplant-ineligible patients, which represent over 70% of patients starting therapy, was initiated in Q4-2024.4 Interim efficacy and safety data for Blenrep as a first line treatment are expected in early 2028 with enrolment expanded to US sites to increase US patient representation in the study population. GSK continues to work with the FDA for US patients.
Blenrep combinations are approved in 2L+ relapsed or refractory multiple myeloma in the European Union7, UK8, Japan9, Canada, Switzerland and Brazil. Applications are currently under review in other markets globally, including China10 where the application is based on the results of DREAMM-7 and has been granted Breakthrough Therapy Designation and Priority Review.
Multiple myeloma is the third most common blood cancer globally and is generally considered treatable but not curable.11,12 There are approximately 180,000 new cases of multiple myeloma diagnosed globally each year. Research into new therapies is needed as multiple myeloma commonly becomes refractory to available treatments.13 Many patients with multiple myeloma, including approximately 70% in the US, are treated in a community cancer setting, leaving an urgent need for new, effective therapies with manageable side effects that can be administered outside of an academic centre.3,15,16
Blenrep is a monoclonal ADC (antibody-drug conjugate) comprising a humanised BCMA (B-cell maturation antigen) conjugated to the cytotoxic agent auristatin F via a non-cleavable linker. The drug linker technology is licensed from Seagen Inc.; the monoclonal antibody is produced using POTELLIGENT Technology licensed from BioWa Inc., a member of the Kyowa Kirin Group.
In the US, Blenrep is indicated in combination with bortezomib and dexamethasone (BVd) for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least two prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent.
Please see accompanying US Prescribing Information which will soon be available here17.
DREAMM-7 is a multicentre, open-label, randomised phase III clinical trial evaluating the efficacy and safety of belantamab mafodotin combined with bortezomib plus dexamethasone (BVd) compared to daratumumab combined with bortezomib plus dexamethasone (DVd) in patients with relapsed or refractory multiple myeloma who previously were treated with at least one prior line of multiple myeloma therapy, with documented disease progression during or after their most recent therapy. The trial enrolled 494 participants who were randomised 1:1 to receive either BVd or DVd. Belantamab mafodotin was administered at a dose of 2.5mg/kg intravenously every three weeks in combination for the first eight cycles and then continued as a single agent. The primary endpoint was PFS as per an independent review committee, with secondary endpoints including OS, duration of response (DOR), and minimal residual disease (MRD) negativity rate as assessed by next-generation sequencing. Other secondary endpoints include overall response rate (ORR), safety, and patient reported and quality of life outcomes.
PFS results17 were presented at the American Society of Clinical Oncology (ASCO) Plenary Series in February 2024 and published in the New England Journal of Medicine. OS results18 were presented at the American Society of Hematology (ASH) Annual Meeting in December 2024.
DREAMM-10 is a multicentre, open-label, randomised phase III clinical trial in newly diagnosed transplant ineligible patients with multiple myeloma, evaluating belantamab mafodotin plus lenalidomide and dexamethasone (BRd) versus daratumumab plus lenalidomide and dexamethasone (DRd).4
Our ambition in oncology is to help increase overall quality of life, maximise survival and change the course of disease, expanding from our current focus on blood and women’s cancers into lung and gastrointestinal cancers, as well as other solid tumours. This includes accelerating priority programmes such as antibody-drug conjugates targeting B7-H3 and B7-H4, and IDRX-42, a highly selective KIT tyrosine kinase inhibitor.
GSK is a global biopharma company with a purpose to unite science, technology, and talent to get ahead of disease together. Find out more at gsk.com.
GSK cautions investors that any forward-looking statements or projections made by GSK, including those made in this announcement, are subject to risks and uncertainties that may cause actual results to differ materially from those projected. Such factors include, but are not limited to, those described in the “Risk Factors” section in GSK’s Annual Report on Form 20-F for 2024, and GSK’s Q2 Results for 2025.

Aston Villa boss Unai Emery said his side have to become more clinical from the penalty spot after a shock Europa League defeat to Dutch minnows Go Ahead Eagles.
Emiliano Buendía missed a late spot-kick as Villa…

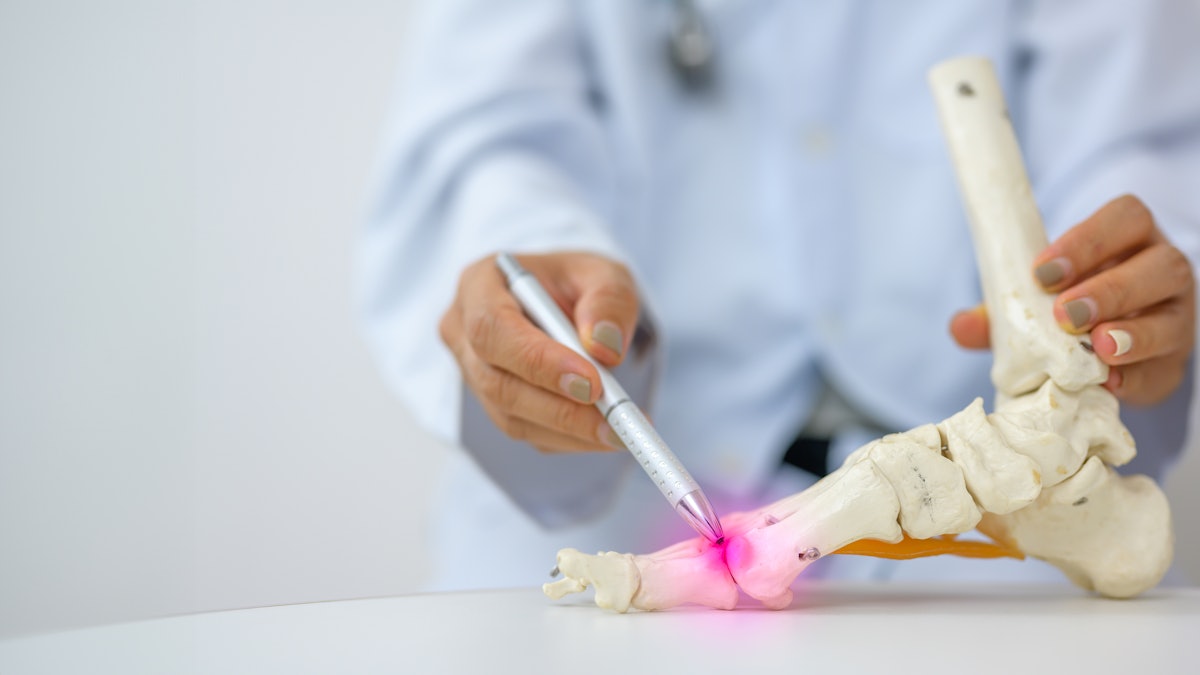
Interventional radiologists in Turkey have reported success with an emerging minimally invasive procedure for treating chronic pain in toe joints due to inflammatory arthritis, gout, or osteoarthritis.
The procedure is called transarterial…

The US Department of Agriculture’s (USDA’s) Animal and Plant Health Inspection Service (APHIS) announced another flurry of highly pathogenic avian flu detections in waterfowl and other wild birds from across the country.
APHIS also reported that…

Traders work on the floor of the New York Stock Exchange during afternoon trading on Oct. 14, 2025 in New York City.
Michael M. Santiago | Getty Images
The S&P 500 rose on Thursday, boosted by tech stocks, as investors stepped in to buy after a batch of strong earnings results.
The broad market index climbed 0.58% to close at 6,738.44, while the Dow Jones Industrial Average traded up 144.20 points, or 0.31%, to finish at 46,734.61. The Nasdaq Composite outperformed, rising 0.89% to settle at 22,941.80, seeing support from the gains in names like Nvidia, Broadcom and Amazon. A nearly 3% jump in shares of fellow artificial intelligence player Oracle also helped send the market higher.
Averages hit their highs of the session after White House press secretary Karoline Leavitt said during a press briefing that President Donald Trump will meet with Chinese President Xi Jinping next Thursday in South Korea. The announcement eased investors’ fears about U.S.-China relations that had pressured equities on Wednesday.
The S&P 500’s move higher marks a full recovery and more from its meaningful losses seen in the previous session, when the index fell roughly 0.5%. The Dow lost about 334 points, or 0.7%, while the Nasdaq declined 0.9% as investors rotated out of riskier assets.
Stocks had finished lower Wednesday after Treasury Secretary Scott Bessent confirmed the White House is mulling plans to curb exports to China made with U.S. software. Those plans would build on Trump’s statement almost two weeks ago that the U.S. will implement export restrictions by Nov. 1 on “any and all critical software.”
“Do not discount the bull market yet, just because of a volatility bout,” said Giuseppe Sette, co-founder and president at Reflexivity. “A handful of tech stocks have led the rally, but now we stand to see how hundreds of global companies benefit from AI’s productivity gains.”
Investors are continuing to watch earnings releases from key U.S. companies, which many believe could be make-or-break for the current bull market rally. Honeywell shares led the blue-chip Dow’s rise, advancing almost 7% Thursday, after it posted better-than-expected quarterly results and lifted its full-year outlook. American Airlines increased 6% following its narrower-than-expected third-quarter loss and upbeat guidance.
The market was able to overcome what had been sore spots in the trading day. Tesla – which kicked off reports from the “Magnificent Seven” – ended up 2% after coming back from earlier losses following a mixed third-quarter report. IBM shares also pared losses after beating Wall Street estimates but reporting in-line software revenue. Meanwhile, oil prices rose after the Trump administration imposed new sanctions on Russia’s two biggest crude companies due to the country’s “lack of serious commitment to a peace process to end the war in Ukraine.”
More than 80% of the S&P 500 companies that have reported so far have exceeded earnings expectations, per FactSet.
“While we are seeing individual stocks get punished after missing expectations, we expect earnings overall to be strong enough to keep stock prices elevated in the near-term,” said Emily Bowersock Hill, CEO and founding partner at Bowersock Capital Partners. “This current earnings season is unlikely to disappoint investors enough to trigger a notable market setback.”
Beyond earnings, inflation data due Friday is expected to give further clues about the health of the economy, particularly ahead of the Federal Reserve’s late October meeting. Markets widely expect central bankers to cut rates by another quarter percentage point.

Unlock the Editor’s Digest for free
Roula Khalaf, Editor of the FT, selects her favourite stories in this weekly newsletter.
Ford has warned of a profit hit of up to $2bn from a fire at an aluminium supplier’s plant in New York, prompting a downgrade in its annual guidance.
The September 16 fire at the plant operated by Novelis has shut down production of aluminium sheets that are widely used by the car industry in the US, including by Ford and Stellantis.
On Thursday, Ford said it expects an adjusted operating profit of $6bn-$6.5bn for the full year, compared with its earlier target range of $6.5bn-$7.5bn. The downgraded forecast included up to $1bn in net tariff impact, which was smaller than the $2bn net hit it projected in July.
Chief financial officer Sherry House said the company would have raised its guidance if not for the cost impact from the Novelis plant fire.
The carmaker said it would add 1,000 jobs across plants in Michigan and Kentucky to increase output of F-series trucks by more than 50,000 vehicles next year to recover production losses caused by the fire.
For the September quarter, Ford reported net income of $2.4bn on a 9 per cent increase in revenue to a record $50.5bn.
Ford’s adjusted earnings of $2.6bn before interest and tax was flat year-on-year, but higher than the average analyst estimate for $2bn, according to Visible Alpha. For the quarter, it booked a tariff impact of $700mn.